Abstract
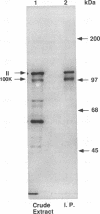
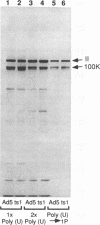
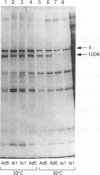
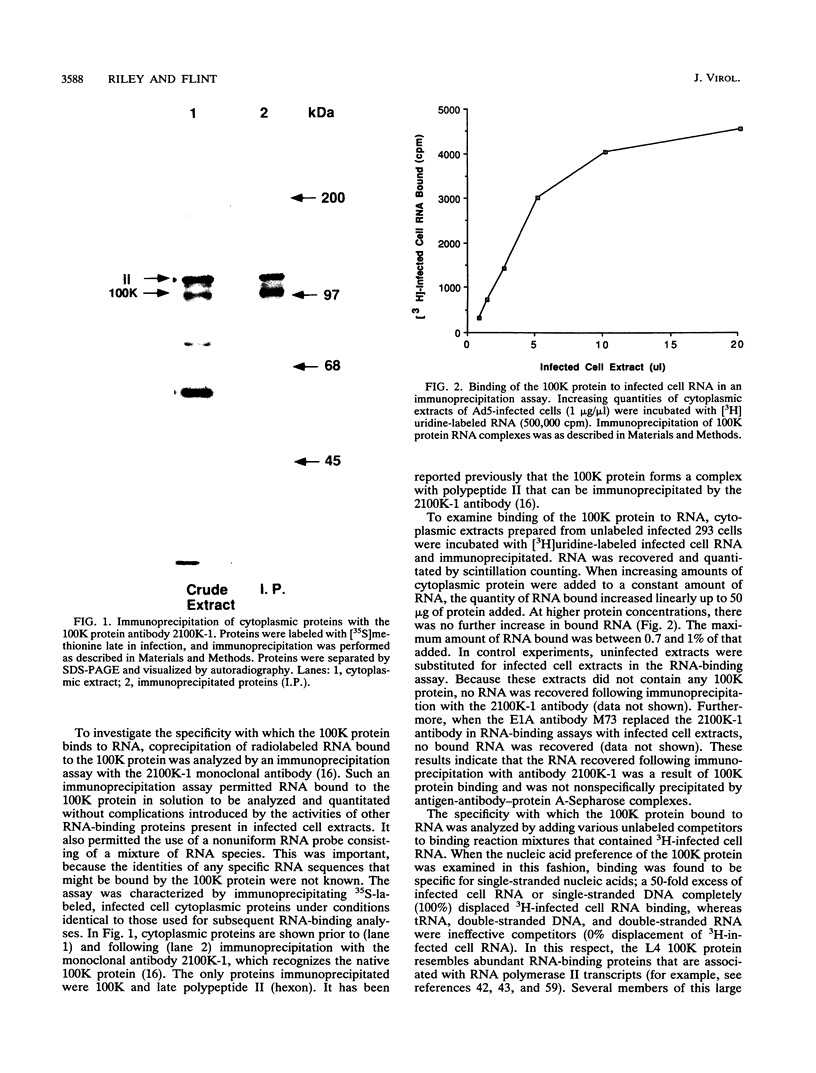
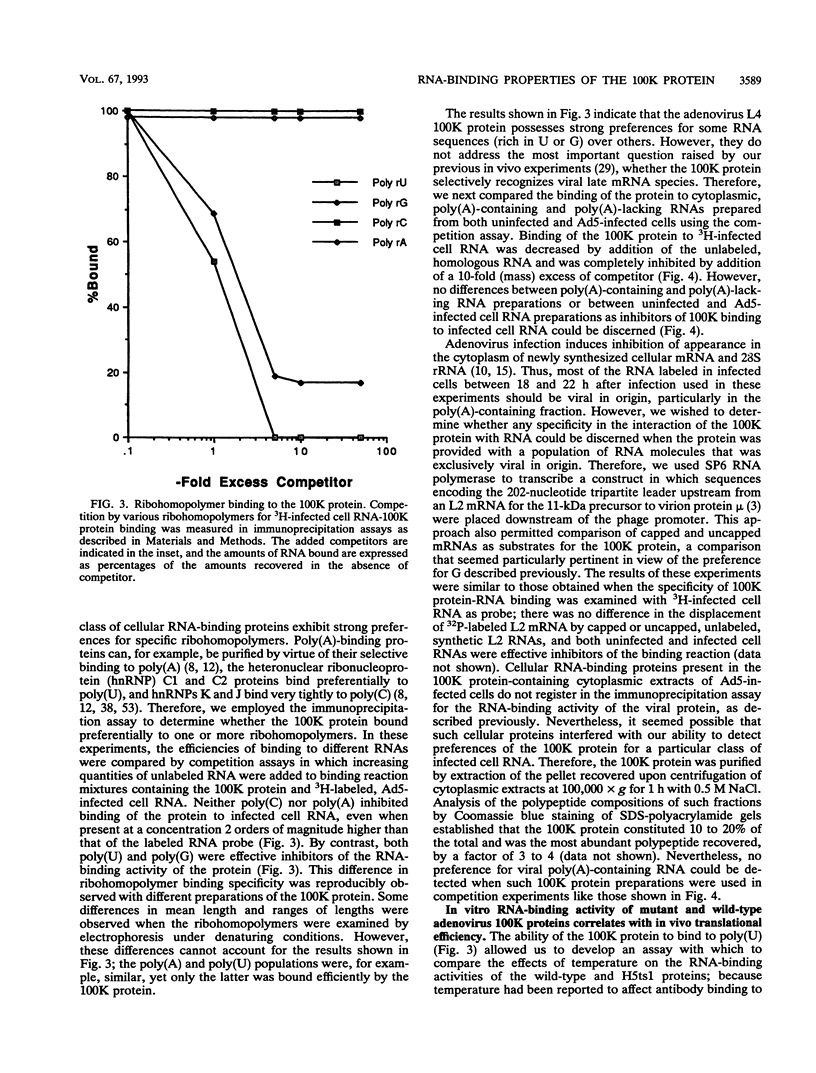
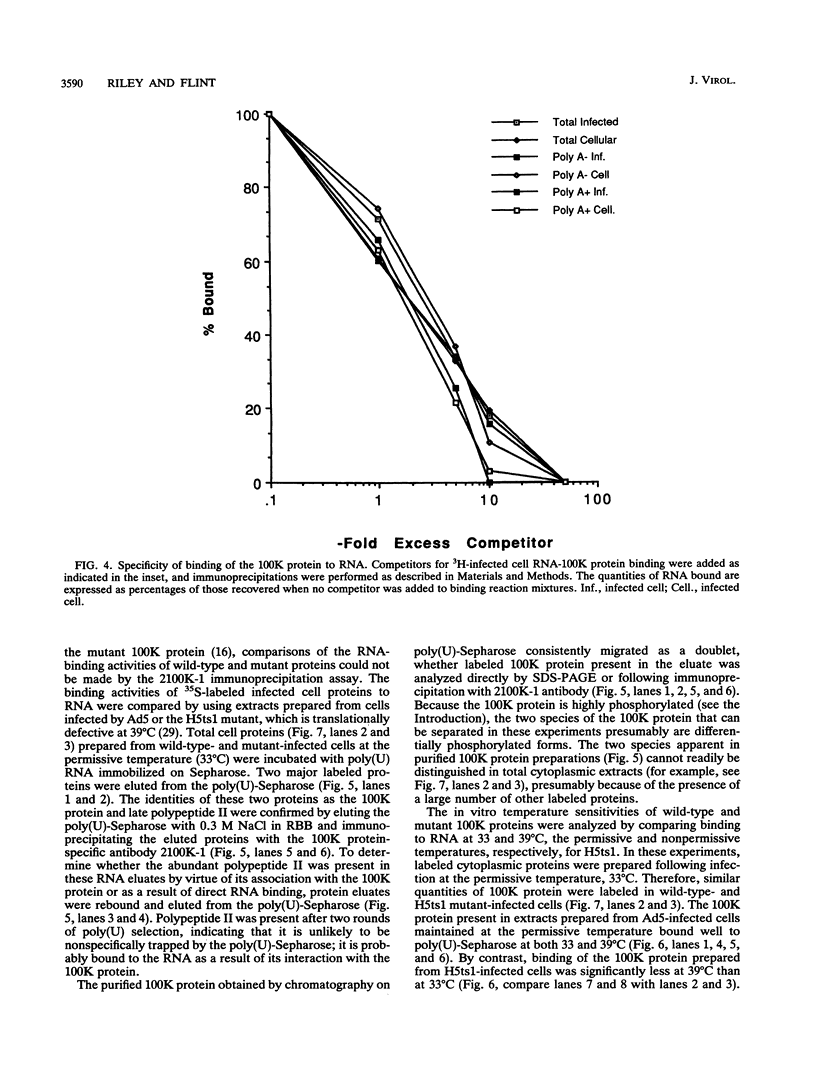
The adenovirus L4 100-kDa nonstructural protein (100K protein) is required for efficient initiation of translation of viral late mRNA species during the late mRNA species during the late phase of infection (B. W. Hayes, G. C. Telling, M. M. Myat, J. F. Williams, and S. J. Flint, J. Virol. 64:2732-2742, 1990). The RNA-binding properties of this protein were analyzed in an immunoprecipitation assay with the 100K-specific monoclonal antibody 2100K-1 (C. L. Cepko and P. A. Sharp, Virology 129:137-154, 1983). Coprecipitation of the 100K protein and 3H-infected cell RNA was demonstrated. The RNA-binding activity of the 100K protein was inhibited by single-stranded DNA but not by double-stranded DNA, double-stranded RNA, or tRNA. Competition assays were used to investigate the specificity with which the 100K protein binds to RNA in vitro. Although the protein exhibited a strong preference for the ribohomopolymer poly(U) or poly(G), no specific binding to viral mRNA species could be detected; uninfected or adenovirus type 5-infected HeLa cell poly(A)-containing and poly(A)-lacking RNAs were all effective inhibitors of binding of the protein to viral late mRNA. Similar results were obtained when the binding of the 100K protein to a single, in vitro-synthesized L2 mRNA was assessed. The poly(U)-binding activity of the 100K protein was used to compare the RNA-binding properties of the 100K protein prepared from cells infected by adenovirus type 5 and the H5ts1 mutant (B. W. Hayes, G. C. Telling, M. M. Myat, J. F. Williams, and S. J. Flint, J. Virol. 64:2732-2742, 1990). A temperature-dependent decrease in H5ts1 100K protein binding was observed, correlating with the impaired translational function of this protein in vivo. By contrast, wild-type 100K protein RNA binding was unaffected by temperature. These data suggest that the 100K protein acts to increase the translational efficiency of viral late mRNA species by a mechanism that involves binding to RNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Dreyfuss G. Adenovirus proteins associated with mRNA and hnRNA in infected HeLa cells. J Virol. 1987 Oct;61(10):3276–3283. doi: 10.1128/jvi.61.10.3276-3283.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Caplen F. V., Katze M. G., Krug R. M. Efficient transcription, not translation, is dependent on adenovirus tripartite leader sequences at late times of infection. J Virol. 1988 May;62(5):1606–1616. doi: 10.1128/jvi.62.5.1606-1616.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Young M. E., Flint S. J. Characterization of the adenovirus 2 virion protein, mu. Virology. 1989 Oct;172(2):506–512. doi: 10.1016/0042-6822(89)90193-1. [DOI] [PubMed] [Google Scholar]
- Arrigo S. J., Chen I. S. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu 2 RNAs. Genes Dev. 1991 May;5(5):808–819. doi: 10.1101/gad.5.5.808. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B. W., Kornberg R. D. The protein responsible for the repeating structure of cytoplasmic poly(A)-ribonucleoprotein. J Cell Biol. 1983 Mar;96(3):717–721. doi: 10.1083/jcb.96.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Effect of the tripartite leader on synthesis of a non-viral protein in an adenovirus 5 recombinant. Nucleic Acids Res. 1985 Feb 11;13(3):841–857. doi: 10.1093/nar/13.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J. M., Brissac C., Jeanteur P. Characterization of a protein species isolated from HeLa cell cytoplasm by affinity chromatography on polyadenylate-sepharose. Proc Natl Acad Sci U S A. 1974 May;71(5):1882–1886. doi: 10.1073/pnas.71.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge E., Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990 Feb;174(2):345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- Castiglia C. L., Flint S. J. Effects of adenovirus infection on rRNA synthesis and maturation in HeLa cells. Mol Cell Biol. 1983 Apr;3(4):662–671. doi: 10.1128/mcb.3.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Sharp P. A. Analysis of Ad5 hexon and 100K ts mutants using conformation-specific monoclonal antibodies. Virology. 1983 Aug;129(1):137–154. doi: 10.1016/0042-6822(83)90402-6. [DOI] [PubMed] [Google Scholar]
- Choi Y. D., Grabowski P. J., Sharp P. A., Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986 Mar 28;231(4745):1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- Cutt J. R., Shenk T., Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987 Feb;61(2):543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino D. M., Felber B. K., Harrison J. E., Pavlakis G. N. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol Cell Biol. 1992 Mar;12(3):1375–1386. doi: 10.1128/mcb.12.3.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph P. J., Racaniello V., Villamarin A., Palladino F., Schneider R. J. The adenovirus tripartite leader may eliminate the requirement for cap-binding protein complex during translation initiation. J Virol. 1988 Jun;62(6):2059–2066. doi: 10.1128/jvi.62.6.2059-2066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Vazeux R., Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell. 1989 Jun 30;57(7):1155–1165. doi: 10.1016/0092-8674(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Hadzopoulou-Cladaras M., Cladaras C., Copeland T., Pavlakis G. N. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambke C., Deppert W. Late nonstructural 100,000- and 33,000-dalton proteins of adenovirus type 2. I. Subcellular localization during the course of infection. J Virol. 1981 Nov;40(2):585–593. doi: 10.1128/jvi.40.2.585-593.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambke C., Deppert W. Specific complex of the late nonstructural 100,000-dalton protein with newly synthesized hexon in adenovirus type 2-infected cells. Virology. 1983 Jan 15;124(1):1–12. doi: 10.1016/0042-6822(83)90285-4. [DOI] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hayes B. W., Telling G. C., Myat M. M., Williams J. F., Flint S. J. The adenovirus L4 100-kilodalton protein is necessary for efficient translation of viral late mRNA species. J Virol. 1990 Jun;64(6):2732–2742. doi: 10.1128/jvi.64.6.2732-2742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. T., Schneider R. J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap-binding protein. Cell. 1991 Apr 19;65(2):271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- Huang J. T., Schneider R. J. Adenovirus inhibition of cellular protein synthesis is prevented by the drug 2-aminopurine. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7115–7119. doi: 10.1073/pnas.87.18.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., DeCorato D., Safer B., Galabru J., Hovanessian A. G. Adenovirus VAI RNA complexes with the 68 000 Mr protein kinase to regulate its autophosphorylation and activity. EMBO J. 1987 Mar;6(3):689–697. doi: 10.1002/j.1460-2075.1987.tb04809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974 Jun 25;86(2):451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991 Nov;65(11):5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis M. J., Michael W. M., Dreyfuss G. Characterization and primary structure of the poly(C)-binding heterogeneous nuclear ribonucleoprotein complex K protein. Mol Cell Biol. 1992 Jan;12(1):164–171. doi: 10.1128/mcb.12.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley R. P., Duncan R. F., Hershey J. W., Mathews M. B. Modification of protein synthesis initiation factors and the shut-off of host protein synthesis in adenovirus-infected cells. Virology. 1989 Jan;168(1):112–118. doi: 10.1016/0042-6822(89)90409-1. [DOI] [PubMed] [Google Scholar]
- Oosterom-Dragon E. A., Ginsberg H. S. Characterization of two temperature-sensitive mutants of type 5 adenovirus with mutations in the 100,000-dalton protein gene. J Virol. 1981 Nov;40(2):491–500. doi: 10.1128/jvi.40.2.491-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S., Moore M., Logan J., Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986 Feb;6(2):470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñol-Roma S., Swanson M. S., Matunis M. J., Dreyfuss G. Purification and characterization of proteins of heterogeneous nuclear ribonucleoprotein complexes by affinity chromatography. Methods Enzymol. 1990;181:326–331. doi: 10.1016/0076-6879(90)81133-f. [DOI] [PubMed] [Google Scholar]
- Pullman J. M., Martin T. E. Reconstitution of nucleoprotein complexes with mammalian heterogeneous nuclear ribonucleoprotein (hnRNP) core proteins. J Cell Biol. 1983 Jul;97(1):99–111. doi: 10.1083/jcb.97.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Sierakowska H., Szer W., Furdon P. J., Kole R. Antibodies to hnRNP core proteins inhibit in vitro splicing of human beta-globin pre-mRNA. Nucleic Acids Res. 1986 Jul 11;14(13):5241–5254. doi: 10.1093/nar/14.13.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist B., Persson T., Lindberg U. Characterization of mRNA-protein complexes from mammalian cells. Nucleic Acids Res. 1977 Apr;4(4):899–915. doi: 10.1093/nar/4.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. RNA binding specificity of hnRNP proteins: a subset bind to the 3' end of introns. EMBO J. 1988 Nov;7(11):3519–3529. doi: 10.1002/j.1460-2075.1988.tb03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasseron-De Jong J. G., Brouwer J., Rietveld K., Zoetemelk C. E., Bosch L. Messenger ribonucleoprotein complexes in human KB cells infected with adenovirus type 5 contain tightly bound viral-coded '100K' proteins. Eur J Biochem. 1979 Oct;100(1):271–283. doi: 10.1111/j.1432-1033.1979.tb02058.x. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. Alterations of transcription and translation in HeLa cells exposed to amino acid analogs. Mol Cell Biol. 1984 Jun;4(6):1063–1072. doi: 10.1128/mcb.4.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Marel P., Tasseron-de Jong J. G., Bosch L. The proteins associated with mRNA from uninfected and adenovirus type 5-infected KB cells. FEBS Lett. 1975 Mar 1;51(1):330–334. doi: 10.1016/0014-5793(75)80919-7. [DOI] [PubMed] [Google Scholar]
- Wilk H. E., Angeli G., Schäfer K. P. In vitro reconstitution of 35S ribonucleoprotein complexes. Biochemistry. 1983 Sep 13;22(19):4592–4600. doi: 10.1021/bi00288a038. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Gharpure M., Ustacelebi S., McDonald S. Isolation of temperature-sensitive mutants of adenovirus type 5. J Gen Virol. 1971 May;11(2):95–101. doi: 10.1099/0022-1317-11-2-95. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Feig D. I., Shenk T. The C proteins of heterogeneous nuclear ribonucleoprotein complexes interact with RNA sequences downstream of polyadenylation cleavage sites. Mol Cell Biol. 1988 Oct;8(10):4477–4483. doi: 10.1128/mcb.8.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Shenk T. A uridylate tract mediates efficient heterogeneous nuclear ribonucleoprotein C protein-RNA cross-linking and functionally substitutes for the downstream element of the polyadenylation signal. Mol Cell Biol. 1990 Dec;10(12):6397–6407. doi: 10.1128/mcb.10.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]