Abstract
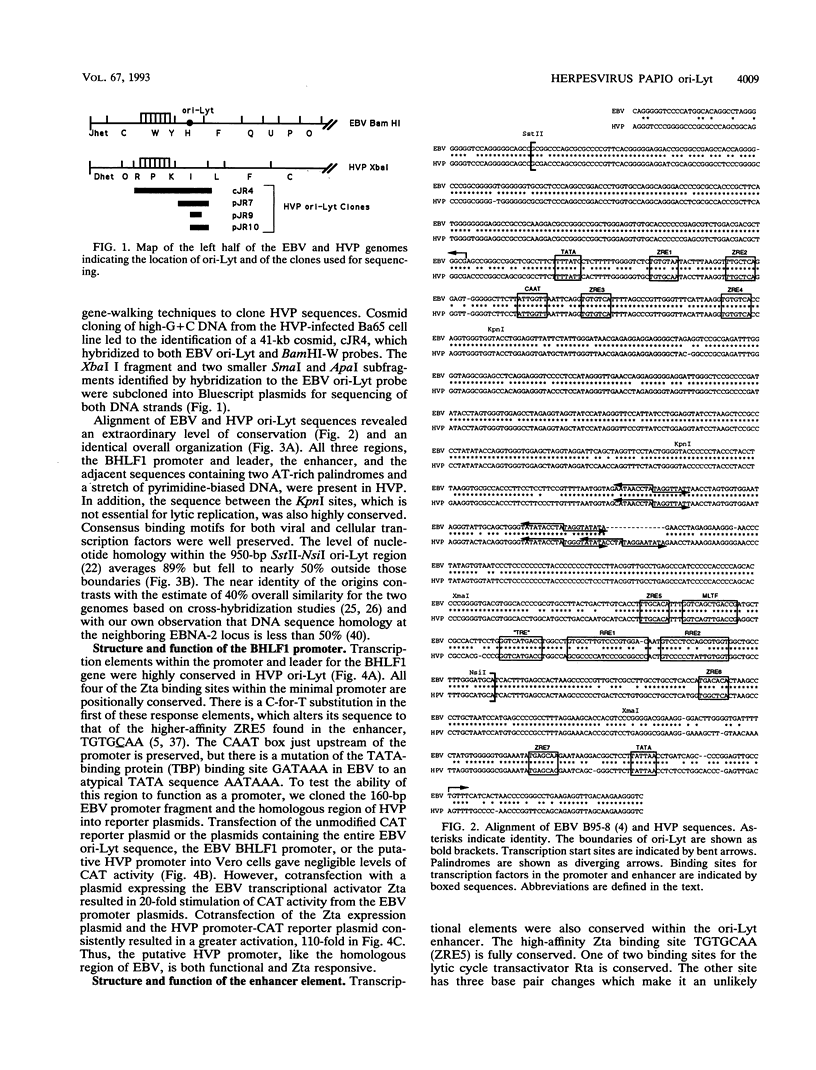
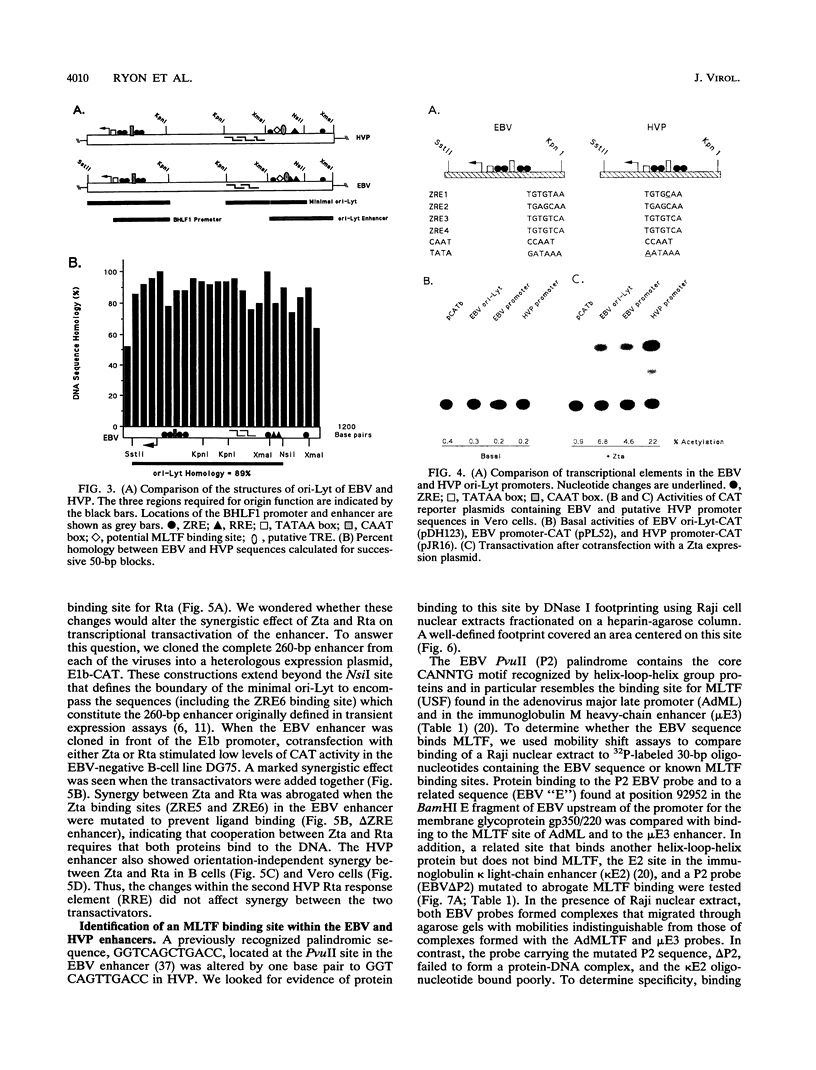
Herpesvirus papio (HVP) is a B-lymphotropic baboon virus with an estimated 40% homology to Epstein-Barr virus (EBV). We have cloned and sequenced ori-Lyt of herpesvirus papio and found a striking degree of nucleotide homology (89%) with ori-Lyt of EBV. Transcriptional elements form an integral part of EBV ori-Lyt. The promoter and enhancer domains of EBV ori-Lyt are conserved in herpesvirus papio. The EBV ori-Lyt promoter contains four binding sites for the EBV lytic cycle transactivator Zta, and the enhancer includes one Zta and two Rta response elements. All five of the Zta response elements and one of the Rta motifs are conserved in HVP ori-Lyt, and the HVP DS-L leftward promoter and the enhancer were activated in transient transfection assays by the EBV Zta and Rta transactivators. The EBV ori-Lyt enhancer contains a palindromic sequence, GGTCAGCTGACC, centered on a PvuII restriction site. This sequence, with a single base change, is also present in the HVP ori-Lyt enhancer. DNase I footprinting demonstrated that the PvuII sequence was bound by a protein present in a Raji nuclear extract. Mobility shift and competition assays using oligonucleotide probes identified this sequence as a binding site for the cellular transcription factor MLTF. Mutagenesis of the binding site indicated that MLTF contributes significantly to the constitutive activity of the ori-Lyt enhancer. The high degree of conservation of cis-acting signal sequences in HVP ori-Lyt was further emphasized by the finding that an HVP ori-Lyt-containing plasmid was replicated in Vero cells by a set of cotransfected EBV replication genes. The central domain of EBV ori-Lyt contains two related AT-rich palindromes, one of which is partially duplicated in the HVP sequence. The AT-rich palindromes are functionally important cis-acting motifs. Deletion of these palindromes severely diminished replication of an ori-Lyt target plasmid.
Full text
PDF


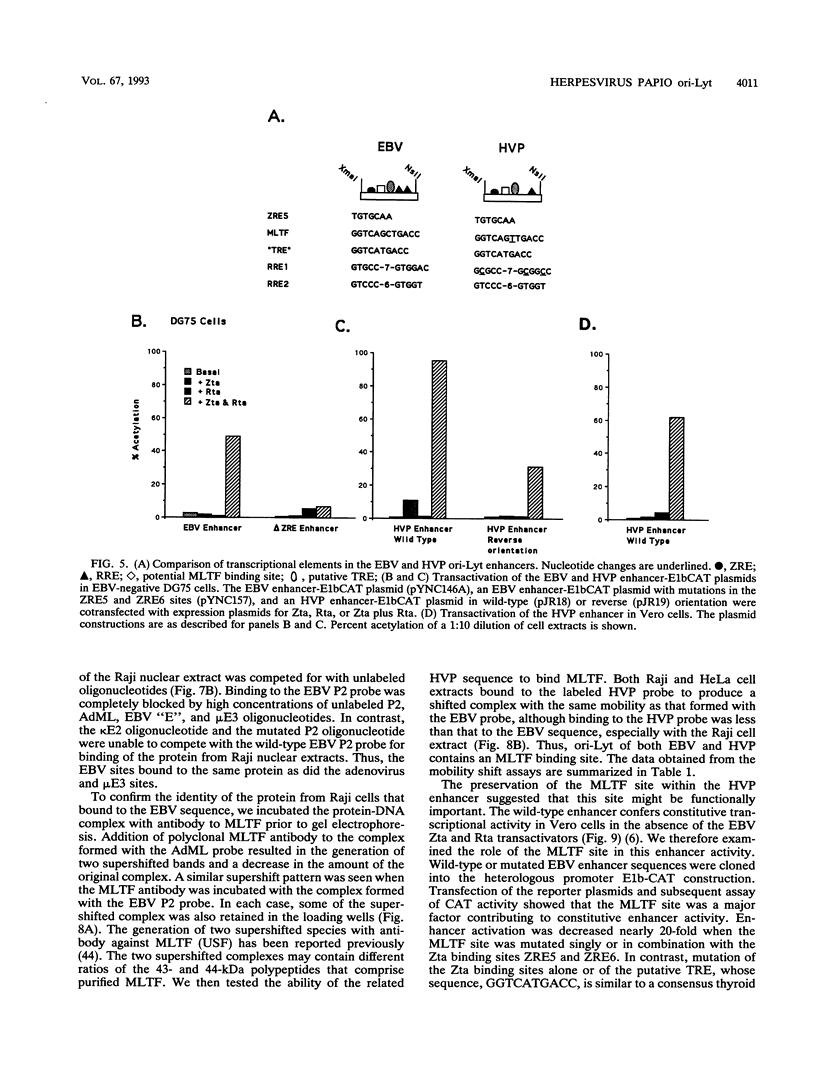

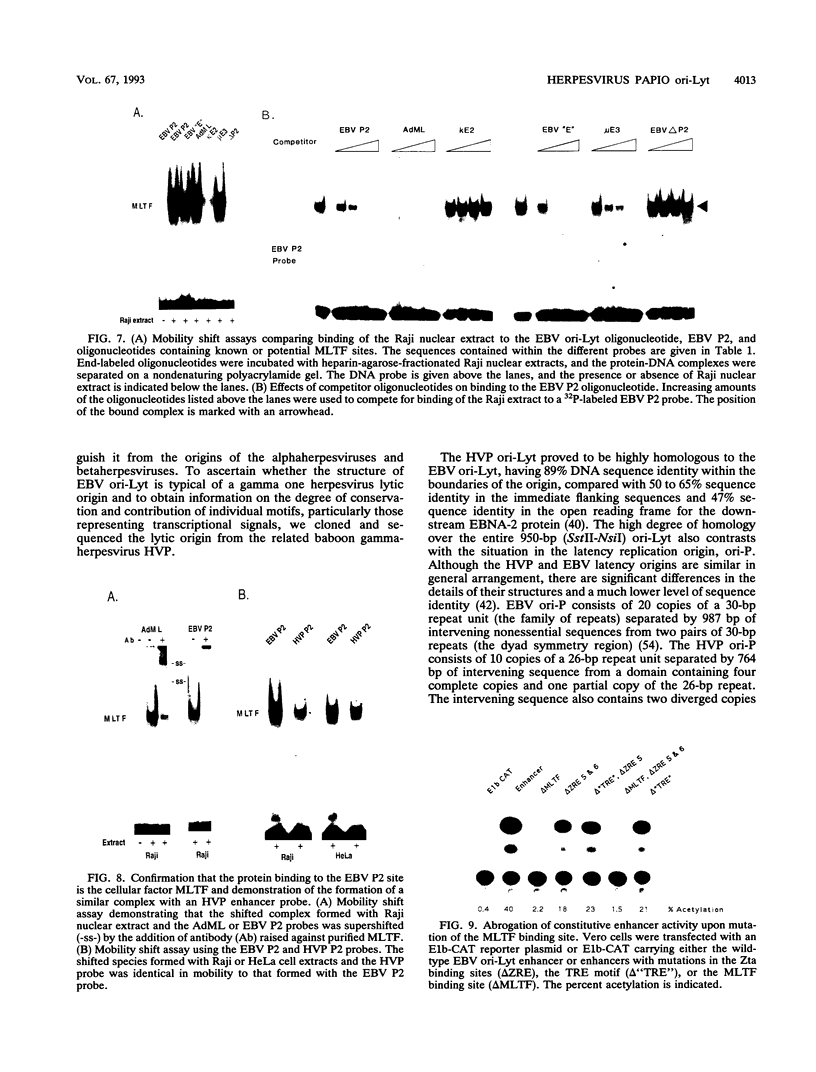
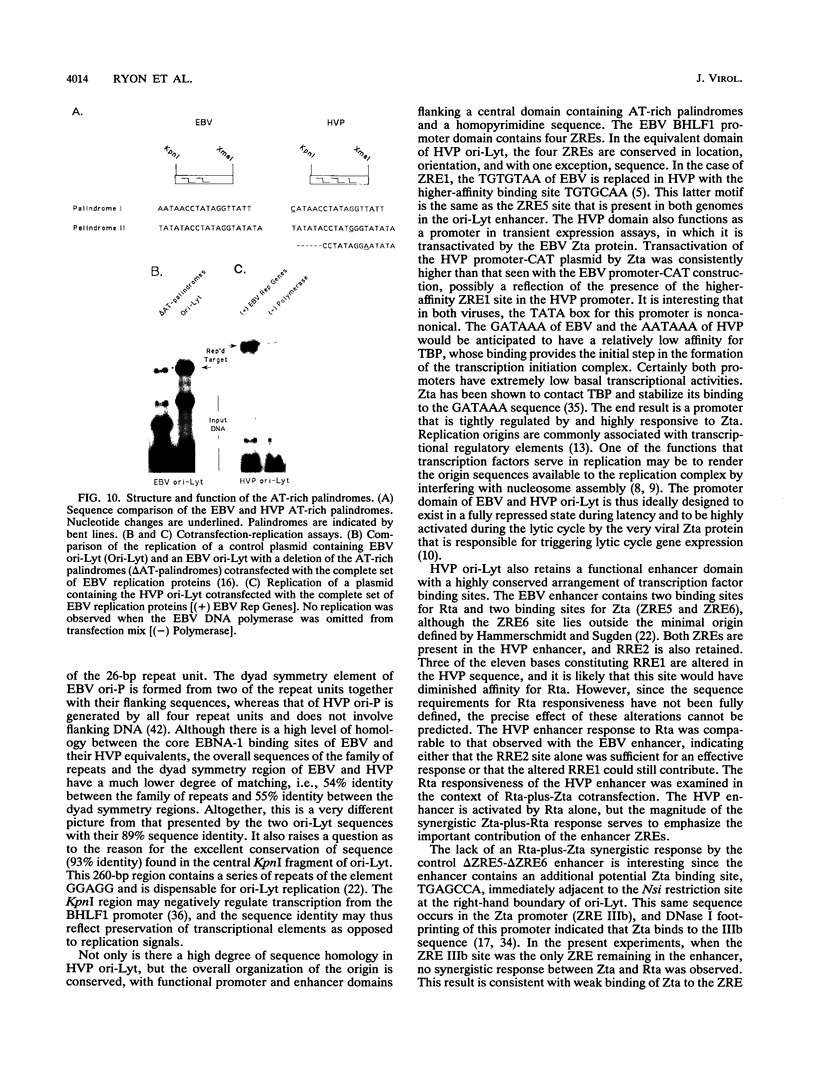


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht J. C., Nicholas J., Biller D., Cameron K. R., Biesinger B., Newman C., Wittmann S., Craxton M. A., Coleman H., Fleckenstein B. Primary structure of the herpesvirus saimiri genome. J Virol. 1992 Aug;66(8):5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders D. G., Kacica M. A., Pari G., Punturieri S. M. Boundaries and structure of human cytomegalovirus oriLyt, a complex origin for lytic-phase DNA replication. J Virol. 1992 Jun;66(6):3373–3384. doi: 10.1128/jvi.66.6.3373-3384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders D. G., Punturieri S. M. Multicomponent origin of cytomegalovirus lytic-phase DNA replication. J Virol. 1991 Feb;65(2):931–937. doi: 10.1128/jvi.65.2.931-937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Chang Y. N., Dong D. L., Hayward G. S., Hayward S. D. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990 Jul;64(7):3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Gruffat H., Chevallier-Greco A., Buisson M., Sergeant A. The Epstein-Barr virus (EBV) early promoter DR contains a cis-acting element responsive to the EBV transactivator EB1 and an enhancer with constitutive and inducible activities. J Virol. 1989 Feb;63(2):607–614. doi: 10.1128/jvi.63.2.607-614.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Cheng L. Z., Workman J. L., Kingston R. E., Kelly T. J. Regulation of DNA replication in vitro by the transcriptional activation domain of GAL4-VP16. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):589–593. doi: 10.1073/pnas.89.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Kelly T. J. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell. 1989 Nov 3;59(3):541–551. doi: 10.1016/0092-8674(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Cox M. A., Leahy J., Hardwick J. M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990 Jan;64(1):313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fixman E. D., Hayward G. S., Hayward S. D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992 Aug;66(8):5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E., Speck S. H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990 Mar;64(3):1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan J. S., Greenspan D., Lennette E. T., Abrams D. I., Conant M. A., Petersen V., Freese U. K. Replication of Epstein-Barr virus within the epithelial cells of oral "hairy" leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985 Dec 19;313(25):1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Gruffat H., Manet E., Rigolet A., Sergeant A. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 1990 Dec 11;18(23):6835–6843. doi: 10.1093/nar/18.23.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S., Gibson W., Hayward G. S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990 Dec;64(12):6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Gerber P., Kieff E. Herpesvirus papio DNA is similar in organization to Epstein-Barr virus DNA. J Virol. 1981 Feb;37(2):698–709. doi: 10.1128/jvi.37.2.698-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Kieff E. Colinearity between the DNAs of Epstein-Barr virus and herpesvirus papio. J Virol. 1981 Feb;37(2):821–826. doi: 10.1128/jvi.37.2.821-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Kieff E. Epstein-Barr virus RNA. VIII. Viral RNA in permissively infected B95-8 cells. J Virol. 1982 Jul;43(1):262–272. doi: 10.1128/jvi.43.1.262-272.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Hayward S. D. Organization of the Epstein-Barr virus DNA molecule. III. Location of the P3HR-1 deletion junction and characterization of the NotI repeat units that form part of the template for an abundant 12-O-tetradecanoylphorbol-13-acetate-induced mRNA transcript. J Virol. 1983 Oct;48(1):135–148. doi: 10.1128/jvi.48.1.135-148.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. H., Hayward S. D., Rawlins D. R. Interaction of the lymphocyte-derived Epstein-Barr virus nuclear antigen EBNA-1 with its DNA-binding sites. J Virol. 1989 Jan;63(1):101–110. doi: 10.1128/jvi.63.1.101-110.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl A., Dorsky D. I. Cooperation of EBV DNA polymerase and EA-D(BMRF1) in vitro and colocalization in nuclei of infected cells. Virology. 1991 Sep;184(1):330–340. doi: 10.1016/0042-6822(91)90849-7. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Tanaka A., Lau R. Y., Nonoyama M., Rabin H. Comparative studies of herpesvirus papio (baboon herpesvirus) DNA and Epstein-Barr virus DNA. J Gen Virol. 1980 Dec;51(Pt 2):245–253. doi: 10.1099/0022-1317-51-2-245. [DOI] [PubMed] [Google Scholar]
- Li J. S., Zhou B. S., Dutschman G. E., Grill S. P., Tan R. S., Cheng Y. C. Association of Epstein-Barr virus early antigen diffuse component and virus-specified DNA polymerase activity. J Virol. 1987 Sep;61(9):2947–2949. doi: 10.1128/jvi.61.9.2947-2949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. In vitro transcriptional activation, dimerization, and DNA-binding specificity of the Epstein-Barr virus Zta protein. J Virol. 1990 Jun;64(6):2560–2568. doi: 10.1128/jvi.64.6.2560-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. The Zta trans-activator protein stabilizes TFIID association with promoter DNA by direct protein-protein interaction. Genes Dev. 1991 Dec;5(12B):2441–2454. doi: 10.1101/gad.5.12b.2441. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Hayward S. D. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J Virol. 1989 Jul;63(7):3040–3050. doi: 10.1128/jvi.63.7.3040-3050.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Sample J., Hayward G. S., Hayward S. D. The zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990 Mar;64(3):1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., O'Hare P., Hayward G. S., Hayward S. D. Promiscuous trans activation of gene expression by an Epstein-Barr virus-encoded early nuclear protein. J Virol. 1986 Oct;60(1):140–148. doi: 10.1128/jvi.60.1.140-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Sista N. D., Besençon F., Kamine J., Pagano J. S. Identification and functional characterization of Epstein-Barr virus DNA polymerase by in vitro transcription-translation of a cloned gene. J Virol. 1991 May;65(5):2728–2731. doi: 10.1128/jvi.65.5.2728-2731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P. D., Ryon J. J., Hayward S. D. EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J Virol. 1993 Jun;67(6):2990–3003. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockshon D., Galloway D. A. Sequence and structural requirements of a herpes simplex viral DNA replication origin. Mol Cell Biol. 1988 Oct;8(10):4018–4027. doi: 10.1128/mcb.8.10.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb D. D., Sung N. S., Pesano R. L., Sexton C. J., Hutchison C., 3rd, Pagano J. S. Plasmid origin of replication of herpesvirus papio: DNA sequence and enhancer function. J Virol. 1990 Jun;64(6):2876–2883. doi: 10.1128/jvi.64.6.2876-2883.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J., Cameron K. R., Coleman H., Newman C., Honess R. W. Analysis of nucleotide sequence of the rightmost 43 kbp of herpesvirus saimiri (HVS) L-DNA: general conservation of genetic organization between HVS and Epstein-Barr virus. Virology. 1992 May;188(1):296–310. doi: 10.1016/0042-6822(92)90759-i. [DOI] [PubMed] [Google Scholar]
- Pognonec P., Roeder R. G. Recombinant 43-kDa USF binds to DNA and activates transcription in a manner indistinguishable from that of natural 43/44-kDa USF. Mol Cell Biol. 1991 Oct;11(10):5125–5136. doi: 10.1128/mcb.11.10.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G. C., Ziff E. B. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science. 1991 Jan 11;251(4990):186–189. doi: 10.1126/science.1987636. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W., Gregor P. D., Roeder R. G. Multiple forms of the human gene-specific transcription factor USF. I. Complete purification and identification of USF from HeLa cell nuclei. J Biol Chem. 1988 Aug 25;263(24):11985–11993. [PubMed] [Google Scholar]
- Stow N. D., McMonagle E. C. Characterization of the TRS/IRS origin of DNA replication of herpes simplex virus type 1. Virology. 1983 Oct 30;130(2):427–438. doi: 10.1016/0042-6822(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Spadaro A., Schaffer J. E., Murray A. W., Maxam A. M., Schaffer P. A. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985 May;5(5):930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- Wong S. W., Schaffer P. A. Elements in the transcriptional regulatory region flanking herpes simplex virus type 1 oriS stimulate origin function. J Virol. 1991 May;65(5):2601–2611. doi: 10.1128/jvi.65.5.2601-2611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]