Abstract
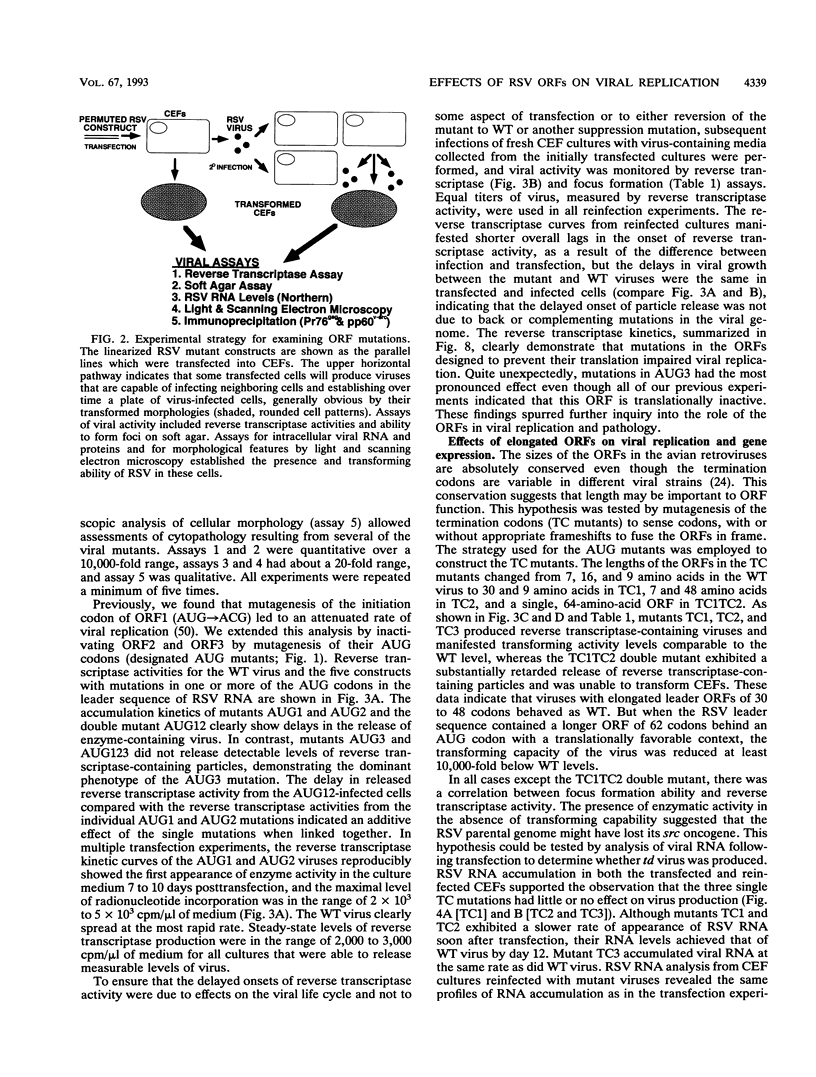
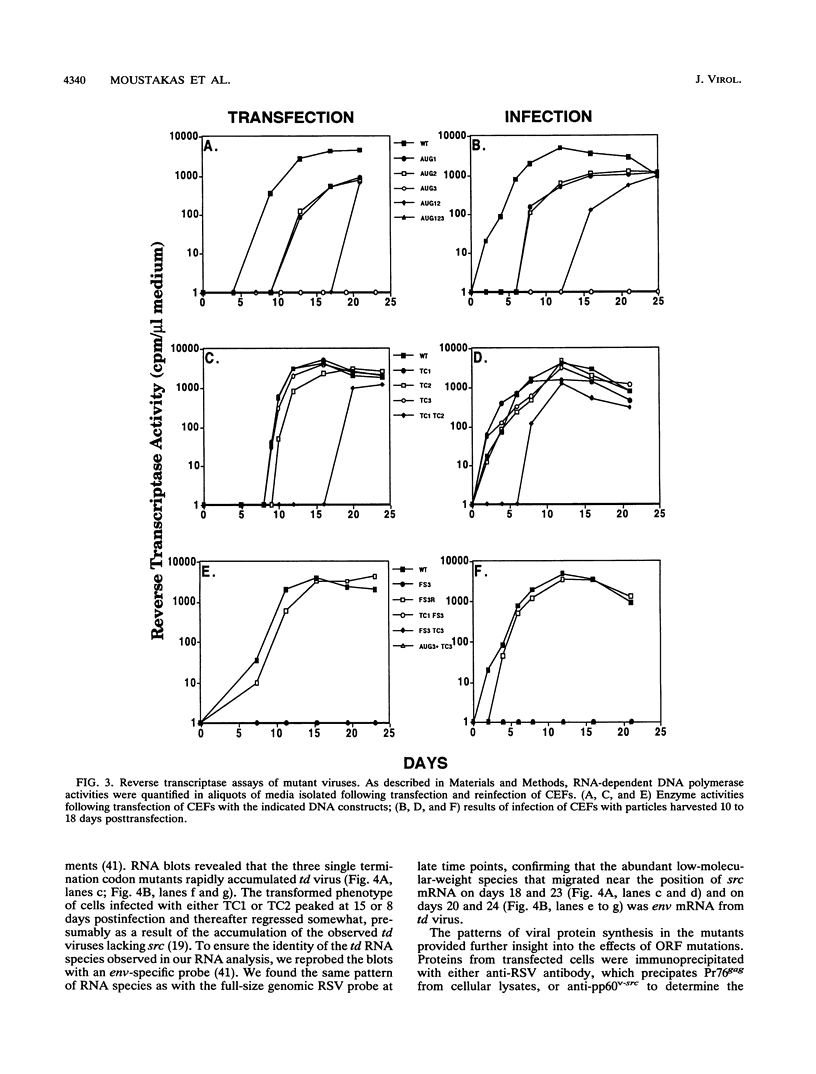
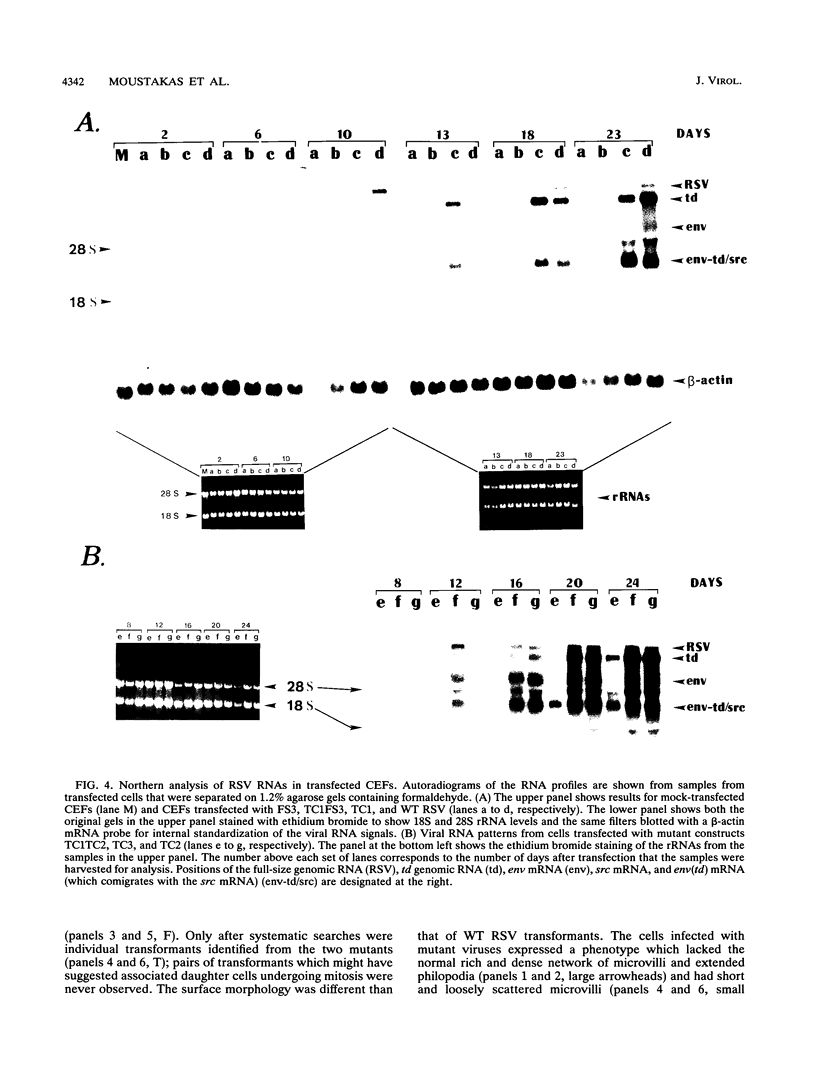
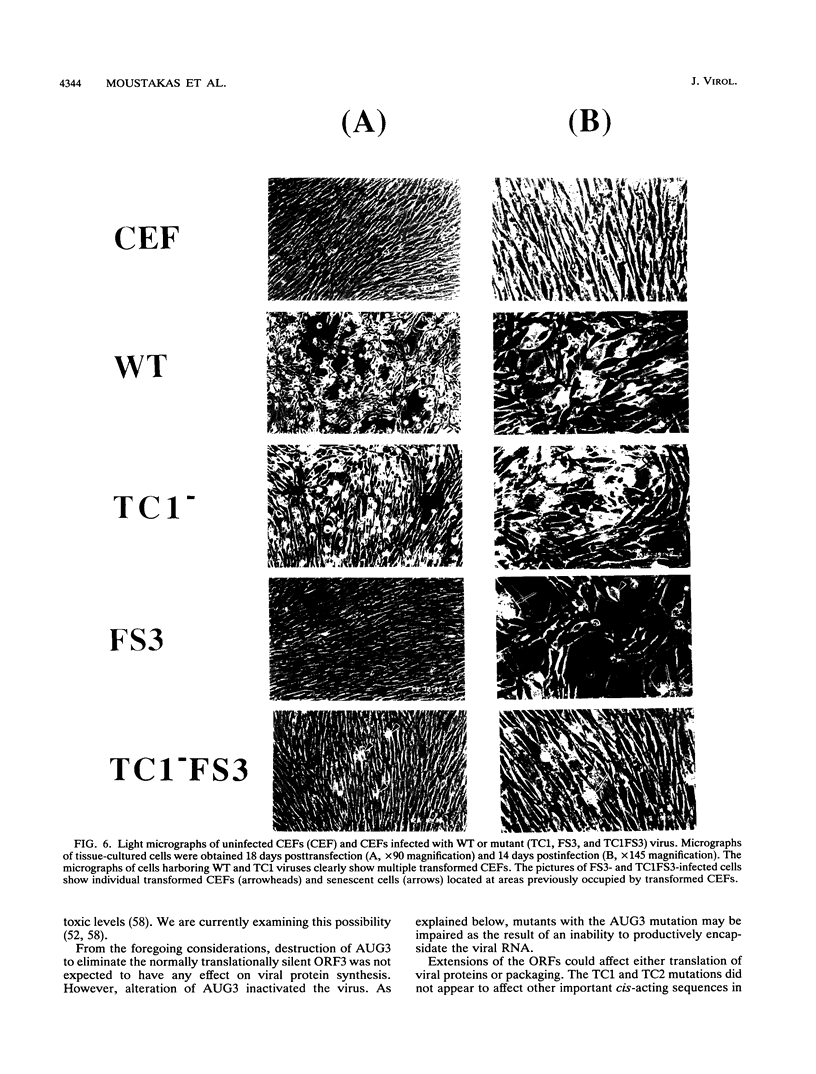
The Rous sarcoma virus (RSV) leader RNA has three short open reading frames (ORF1 to ORF3) which are conserved in all avian sarcoma-leukosis retroviruses. Effects on virus propagation were determined following three types of alterations in the ORFs: (i) replacement of AUG initiation codons in order to prohibit ORF translation, (ii) alterations of the codon context around the AUG initiation codon to enhance translation of the normally silent ORF3, and (iii) elongation of the ORF coding sequences. Mutagenesis of the AUG codons for ORF1 and ORF2 (AUG1 and AUG2) singly or together delayed the onset of viral replication and cell transformation. In contrast, mutagenesis of AUG3 almost completely suppressed these viral activities. Mutagenesis of ORF3 to enhance its translation inhibited viral propagation. When the mutant ORF3 included an additional frameshift mutation which extended the ORF beyond the initiation site for the gag, gag-pol, and env proteins, host cells were initially transformed but died soon thereafter. Elongation of ORF1 from 7 to 62 codons led to the accumulation of transformation-defective virus with a delayed onset of replication. In contrast, viruses with elongation of ORF1 from 7 to 30 codons, ORF2 from 16 to 48 codons, or ORF3 from 9 to 64 codons, without any alterations in the AUG context, exhibited wild-type phenotypes. These results are consistent with a model that translation of the ORFs is necessary to facilitate virus production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar A., Cobrinik D., Ge Z., Kung H. J., Leis J. Interaction between retroviral U5 RNA and the T psi C loop of the tRNA(Trp) primer is required for efficient initiation of reverse transcription. J Virol. 1992 Apr;66(4):2464–2472. doi: 10.1128/jvi.66.4.2464-2472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. R., Chen L. B., Buchanan J. M. Surface ruffles as markers for studies of cell transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3144–3148. doi: 10.1073/pnas.72.8.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Lee P., Levine K. L., Sang J. S., Shah S. A., Yang O. O., Shank P. R., Linial M. L. Molecular cloning and characterization of the RNA packaging-defective retrovirus SE21Q1b. J Virol. 1992 Jan;66(1):204–216. doi: 10.1128/jvi.66.1.204-216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff R., Linial M. Specificity of retroviral RNA packaging. J Virol. 1991 Jan;65(1):71–80. doi: 10.1128/jvi.65.1.71-80.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G. F., Beemon K. Nonsense codons within the Rous sarcoma virus gag gene decrease the stability of unspliced viral RNA. Mol Cell Biol. 1991 May;11(5):2760–2768. doi: 10.1128/mcb.11.5.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieth E., Gabus C., Darlix J. L. A study of the dimer formation of Rous sarcoma virus RNA and of its effect on viral protein synthesis in vitro. Nucleic Acids Res. 1990 Jan 11;18(1):119–127. doi: 10.1093/nar/18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Katz R. A., Skalka A. M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984 Feb;49(2):557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde A., Weiss R. A., Veselý P. Scanning electron microscopy of cells in culture. Exp Cell Res. 1972;71(2):313–324. doi: 10.1016/0014-4827(72)90299-6. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Ganem D., Varmus H. E. Mechanism of translation of the hepadnaviral polymerase (P) gene. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5158–5162. doi: 10.1073/pnas.87.13.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cobrinik D., Soskey L., Leis J. A retroviral RNA secondary structure required for efficient initiation of reverse transcription. J Virol. 1988 Oct;62(10):3622–3630. doi: 10.1128/jvi.62.10.3622-3630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L. Control of Rous sarcoma virus RNA translation and packaging by the 5' and 3' untranslated sequences. J Mol Biol. 1986 Jun 5;189(3):421–434. doi: 10.1016/0022-2836(86)90314-1. [DOI] [PubMed] [Google Scholar]
- Donzé O., Spahr P. F. Role of the open reading frames of Rous sarcoma virus leader RNA in translation and genome packaging. EMBO J. 1992 Oct;11(10):3747–3757. doi: 10.1002/j.1460-2075.1992.tb05460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Dupraz P., Spahr P. F. Specificity of Rous sarcoma virus nucleocapsid protein in genomic RNA packaging. J Virol. 1992 Aug;66(8):4662–4670. doi: 10.1128/jvi.66.8.4662-4670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estis L. F., Temin H. M. Suppression of multiplication of avian sarcoma virus by rapid spread of transformation-defective virus of the same subgroup. J Virol. 1979 Aug;31(2):389–397. doi: 10.1128/jvi.31.2.389-397.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Hohn T. Role of an upstream open reading frame in the translation of polycistronic mRNAs in plant cells. Nucleic Acids Res. 1992 Aug 11;20(15):3851–3857. doi: 10.1093/nar/20.15.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D. A., Sisodia S. S., Cleveland D. W. Autoregulatory control of beta-tubulin mRNA stability is linked to translation elongation. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5763–5767. doi: 10.1073/pnas.86.15.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Dalton M. W., Johnson D. P., Petersen R. B. Phylogenetic and physical analysis of the 5' leader RNA sequences of avian retroviruses. Nucleic Acids Res. 1991 Dec 25;19(24):6929–6934. doi: 10.1093/nar/19.24.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Petersen R. B., Hensel C. H., Albericio F., Gunderson S. I., Palmenberg A. C., Barany G. Synthesis in vitro of a seven amino acid peptide encoded in the leader RNA of Rous sarcoma virus. J Mol Biol. 1986 Jul 5;190(1):45–57. doi: 10.1016/0022-2836(86)90074-4. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Swanstrom R., Varmus H. E., Bishop J. M. The leader sequence of the subgenomic mRNA's of Rous sarcoma virus is approximately 390 nucleotides. J Virol. 1982 Feb;41(2):527–534. doi: 10.1128/jvi.41.2.527-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison G. P., Lever A. M. The human immunodeficiency virus type 1 packaging signal and major splice donor region have a conserved stable secondary structure. J Virol. 1992 Jul;66(7):4144–4153. doi: 10.1128/jvi.66.7.4144-4153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel C. H., Petersen R. B., Hackett P. B. Effects of alterations in the leader sequence of Rous sarcoma virus RNA on initiation of translation. J Virol. 1989 Nov;63(11):4986–4990. doi: 10.1128/jvi.63.11.4986-4990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Mellstrom K., Kosik E., Tamanoi F., Brugge J. Mutation of a termination codon affects src initiation. Mol Cell Biol. 1984 Sep;4(9):1738–1746. doi: 10.1128/mcb.4.9.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E. Biological techniques for avian sarcoma viruses. Methods Enzymol. 1979;58:379–393. doi: 10.1016/s0076-6879(79)58153-1. [DOI] [PubMed] [Google Scholar]
- Johansen H., Schümperli D., Rosenberg M. Affecting gene expression by altering the length and sequence of the 5' leader. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7698–7702. doi: 10.1073/pnas.81.24.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Cullen B. R., Malavarca R., Skalka A. M. Role of the avian retrovirus mRNA leader in expression: evidence for novel translational control. Mol Cell Biol. 1986 Feb;6(2):372–379. doi: 10.1128/mcb.6.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Terry R. W., Skalka A. M. A conserved cis-acting sequence in the 5' leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986 Jul;59(1):163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. A consideration of alternative models for the initiation of translation in eukaryotes. Crit Rev Biochem Mol Biol. 1992;27(4-5):385–402. doi: 10.3109/10409239209082567. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Sonstegard T. S., Hackett P. B. Effects of the open reading frames in the Rous sarcoma virus leader RNA on translation. J Virol. 1993 Jul;67(7):4350–4357. doi: 10.1128/jvi.67.7.4350-4357.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassal M., Junker-Niepmann M., Schaller H. Translational inactivation of RNA function: discrimination against a subset of genomic transcripts during HBV nucleocapsid assembly. Cell. 1990 Dec 21;63(6):1357–1363. doi: 10.1016/0092-8674(90)90431-d. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Koyama T., Kawai S. Unusual features of the leader sequence of Rous sarcoma virus packaging mutant TK15. J Virol. 1985 Sep;55(3):881–885. doi: 10.1128/jvi.55.3.881-885.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle S., Spahr P. F. Role of the gag polyprotein precursor in packaging and maturation of Rous sarcoma virus genomic RNA. J Virol. 1990 Dec;64(12):5757–5763. doi: 10.1128/jvi.64.12.5757-5763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. C., Swanstrom R. A new pathway in the generation of defective retrovirus DNA. J Virol. 1985 Dec;56(3):779–789. doi: 10.1128/jvi.56.3.779-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Hackett P. B. Characterization of ribosome binding on Rous sarcoma virus RNA in vitro. J Virol. 1985 Dec;56(3):683–690. doi: 10.1128/jvi.56.3.683-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Hensel C. H., Hackett P. B. Identification of a ribosome-binding site for a leader peptide encoded by Rous sarcoma virus RNA. J Virol. 1984 Sep;51(3):722–729. doi: 10.1128/jvi.51.3.722-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Moustakas A., Hackett P. B. A mutation in the short 5'-proximal open reading frame on Rous sarcoma virus RNA alters virus production. J Virol. 1989 Nov;63(11):4787–4796. doi: 10.1128/jvi.63.11.4787-4796.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley W. G., Temin H. M. The location of v-src in a retrovirus vector determines whether the virus is toxic or transforming. Mol Cell Biol. 1984 Dec;4(12):2653–2660. doi: 10.1128/mcb.4.12.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]