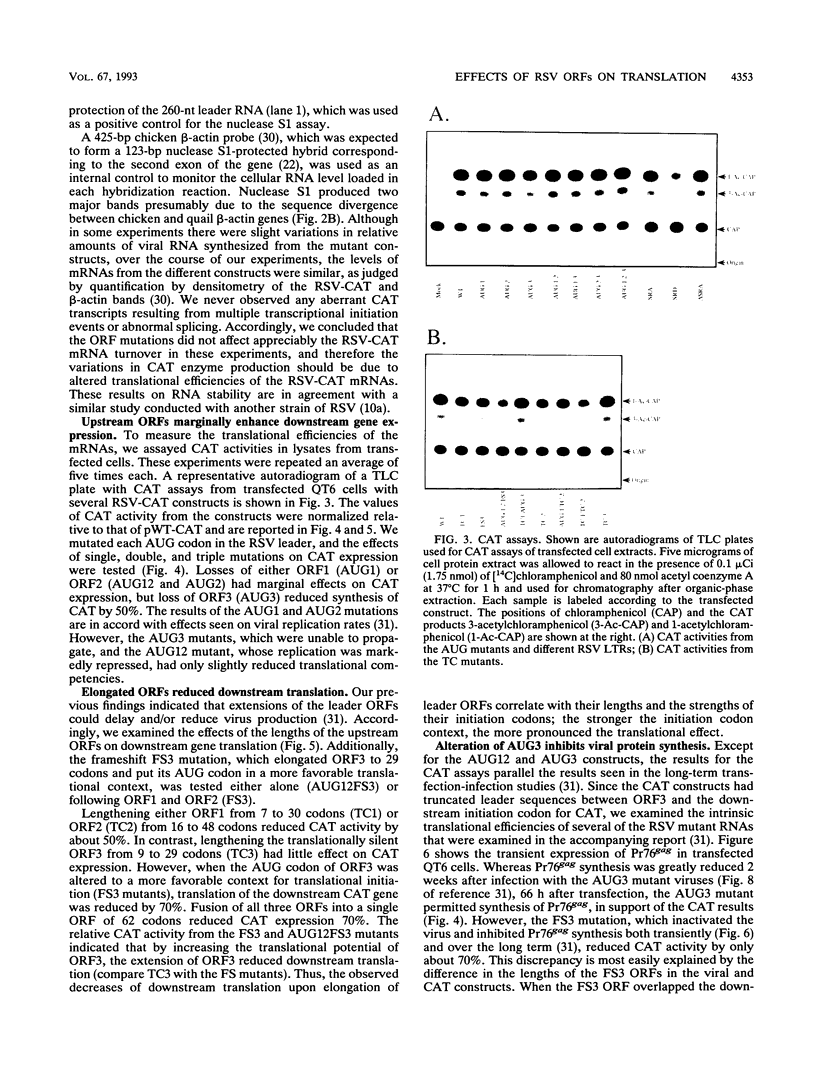
Abstract
Three short open reading frames (ORFs) reside in the 5' leader of Rous sarcoma virus (RSV) and are conserved in all avian sarcoma-leukosis retroviruses. Both extensions of the lengths of the ORFs and alterations in their initiation codons affect viral replication and gene expression. To determine whether the effects on viral replication were due to translational regulation mediated by the ORFs, we examined translation following mutation of the initiation and termination codons of each of the three ORFs. We found that the ORFs marginally enhanced downstream gene expression. Moreover, repression of downstream gene translation was proportional to the lengths of the elongated ORFs and depended on the initiation contexts of the AUG codons. Although the ORFs play a major role in viral activities, their effects on translation were relatively minor. Rather, the ORFs may affect the fate of unspliced avian retroviral RNA in chronically infected cells by participating in the sorting of viral RNA for either translation or encapsidation into virions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff R., Linial M. Specificity of retroviral RNA packaging. J Virol. 1991 Jan;65(1):71–80. doi: 10.1128/jvi.65.1.71-80.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegalke B. J., Geballe A. P. Translational inhibition by cytomegalovirus transcript leaders. Virology. 1990 Aug;177(2):657–667. doi: 10.1016/0042-6822(90)90531-u. [DOI] [PubMed] [Google Scholar]
- Bieth E., Gabus C., Darlix J. L. A study of the dimer formation of Rous sarcoma virus RNA and of its effect on viral protein synthesis in vitro. Nucleic Acids Res. 1990 Jan 11;18(1):119–127. doi: 10.1093/nar/18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Katz R. A., Skalka A. M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984 Feb;49(2):557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt D., Perdue M. L. Identification of an initial site of interaction and possible helix destabilizing activity preceding initiation of protein synthesis from retrovirus RNA. Virus Res. 1988 May;10(2-3):241–248. doi: 10.1016/0168-1702(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cobrinik D., Katz R., Terry R., Skalka A. M., Leis J. Avian sarcoma and leukosis virus pol-endonuclease recognition of the tandem long terminal repeat junction: minimum site required for cleavage is also required for viral growth. J Virol. 1987 Jun;61(6):1999–2008. doi: 10.1128/jvi.61.6.1999-2008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Soskey L., Leis J. A retroviral RNA secondary structure required for efficient initiation of reverse transcription. J Virol. 1988 Oct;62(10):3622–3630. doi: 10.1128/jvi.62.10.3622-3630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L. Control of Rous sarcoma virus RNA translation and packaging by the 5' and 3' untranslated sequences. J Mol Biol. 1986 Jun 5;189(3):421–434. doi: 10.1016/0022-2836(86)90314-1. [DOI] [PubMed] [Google Scholar]
- Donzé O., Spahr P. F. Role of the open reading frames of Rous sarcoma virus leader RNA in translation and genome packaging. EMBO J. 1992 Oct;11(10):3747–3757. doi: 10.1002/j.1460-2075.1992.tb05460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. E. 5'-terminal sequences influence the segregation of ground squirrel hepatitis virus RNAs into polyribosomes and viral core particles. J Virol. 1987 Jan;61(1):35–41. doi: 10.1128/jvi.61.1.35-41.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D., Andrews D. W. Both the 5' untranslated region and the sequences surrounding the start site contribute to efficient initiation of translation in vitro. Mol Cell Biol. 1991 May;11(5):2656–2664. doi: 10.1128/mcb.11.5.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Dalton M. W., Johnson D. P., Petersen R. B. Phylogenetic and physical analysis of the 5' leader RNA sequences of avian retroviruses. Nucleic Acids Res. 1991 Dec 25;19(24):6929–6934. doi: 10.1093/nar/19.24.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Petersen R. B., Hensel C. H., Albericio F., Gunderson S. I., Palmenberg A. C., Barany G. Synthesis in vitro of a seven amino acid peptide encoded in the leader RNA of Rous sarcoma virus. J Mol Biol. 1986 Jul 5;190(1):45–57. doi: 10.1016/0022-2836(86)90074-4. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Swanstrom R., Varmus H. E., Bishop J. M. The leader sequence of the subgenomic mRNA's of Rous sarcoma virus is approximately 390 nucleotides. J Virol. 1982 Feb;41(2):527–534. doi: 10.1128/jvi.41.2.527-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel C. H., Petersen R. B., Hackett P. B. Effects of alterations in the leader sequence of Rous sarcoma virus RNA on initiation of translation. J Virol. 1989 Nov;63(11):4986–4990. doi: 10.1128/jvi.63.11.4986-4990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Cullen B. R., Malavarca R., Skalka A. M. Role of the avian retrovirus mRNA leader in expression: evidence for novel translational control. Mol Cell Biol. 1986 Feb;6(2):372–379. doi: 10.1128/mcb.6.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost T. A., Theodorakis N., Hughes S. H. The nucleotide sequence of the chick cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Dec 10;11(23):8287–8301. doi: 10.1093/nar/11.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. A consideration of alternative models for the initiation of translation in eukaryotes. Crit Rev Biochem Mol Biol. 1992;27(4-5):385–402. doi: 10.3109/10409239209082567. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. J., Moav B., Faras A. J., Guise K. S., Kapuscinski A. R., Hackett P. B. Functional analysis of elements affecting expression of the beta-actin gene of carp. Mol Cell Biol. 1990 Jul;10(7):3432–3440. doi: 10.1128/mcb.10.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A., Sonstegard T. S., Hackett P. B. Alterations of the three short open reading frames in the Rous sarcoma virus leader RNA modulate viral replication and gene expression. J Virol. 1993 Jul;67(7):4337–4349. doi: 10.1128/jvi.67.7.4337-4349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Nassal M., Junker-Niepmann M., Schaller H. Translational inactivation of RNA function: discrimination against a subset of genomic transcripts during HBV nucleocapsid assembly. Cell. 1990 Dec 21;63(6):1357–1363. doi: 10.1016/0092-8674(90)90431-d. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Koyama T., Kawai S. Unusual features of the leader sequence of Rous sarcoma virus packaging mutant TK15. J Virol. 1985 Sep;55(3):881–885. doi: 10.1128/jvi.55.3.881-885.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. C., Swanstrom R. A new pathway in the generation of defective retrovirus DNA. J Virol. 1985 Dec;56(3):779–789. doi: 10.1128/jvi.56.3.779-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin N., Darveau A., Nicholson R., Sonenberg N. cis-acting translational effects of the 5' noncoding region of c-myc mRNA. Mol Cell Biol. 1988 Jul;8(7):2875–2883. doi: 10.1128/mcb.8.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Subramani S., Berg P. Effect of upstream reading frames on translation efficiency in simian virus 40 recombinants. Mol Cell Biol. 1986 Jul;6(7):2704–2711. doi: 10.1128/mcb.6.7.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Hackett P. B. Characterization of ribosome binding on Rous sarcoma virus RNA in vitro. J Virol. 1985 Dec;56(3):683–690. doi: 10.1128/jvi.56.3.683-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Hensel C. H., Hackett P. B. Identification of a ribosome-binding site for a leader peptide encoded by Rous sarcoma virus RNA. J Virol. 1984 Sep;51(3):722–729. doi: 10.1128/jvi.51.3.722-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Moustakas A., Hackett P. B. A mutation in the short 5'-proximal open reading frame on Rous sarcoma virus RNA alters virus production. J Virol. 1989 Nov;63(11):4787–4796. doi: 10.1128/jvi.63.11.4787-4796.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Sonenberg N. Cap-binding proteins of eukaryotic messenger RNA: functions in initiation and control of translation. Prog Nucleic Acid Res Mol Biol. 1988;35:173–207. doi: 10.1016/s0079-6603(08)60614-5. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. A., Scheper G. C., Voorma H. O. Hypothesis: is eukaryotic initiation factor 2 the scanning factor? New Biol. 1992 Apr;4(4):404–407. [PubMed] [Google Scholar]