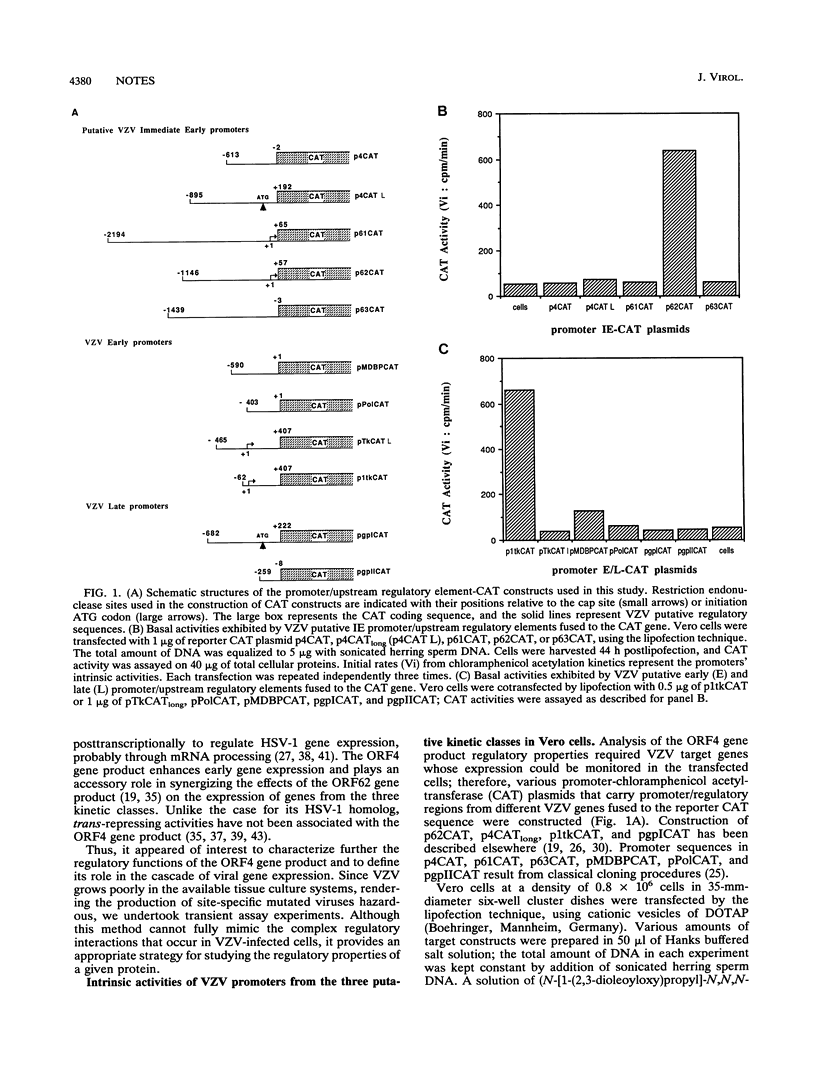
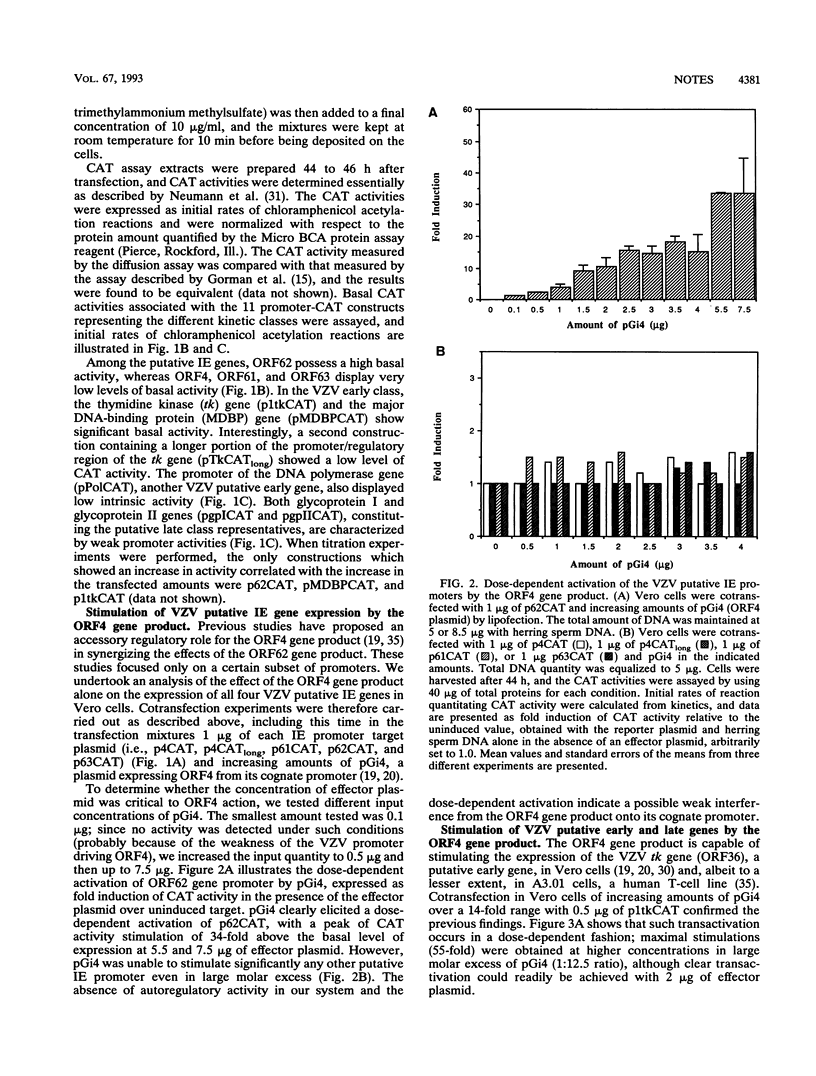
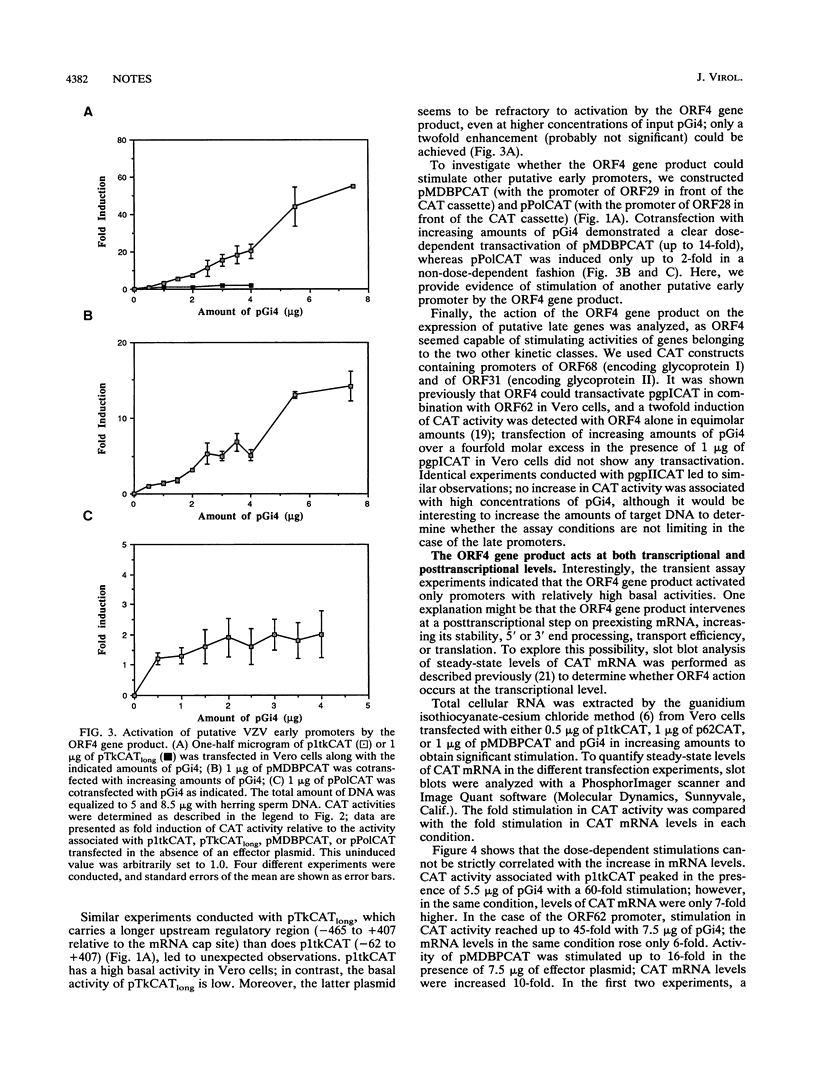
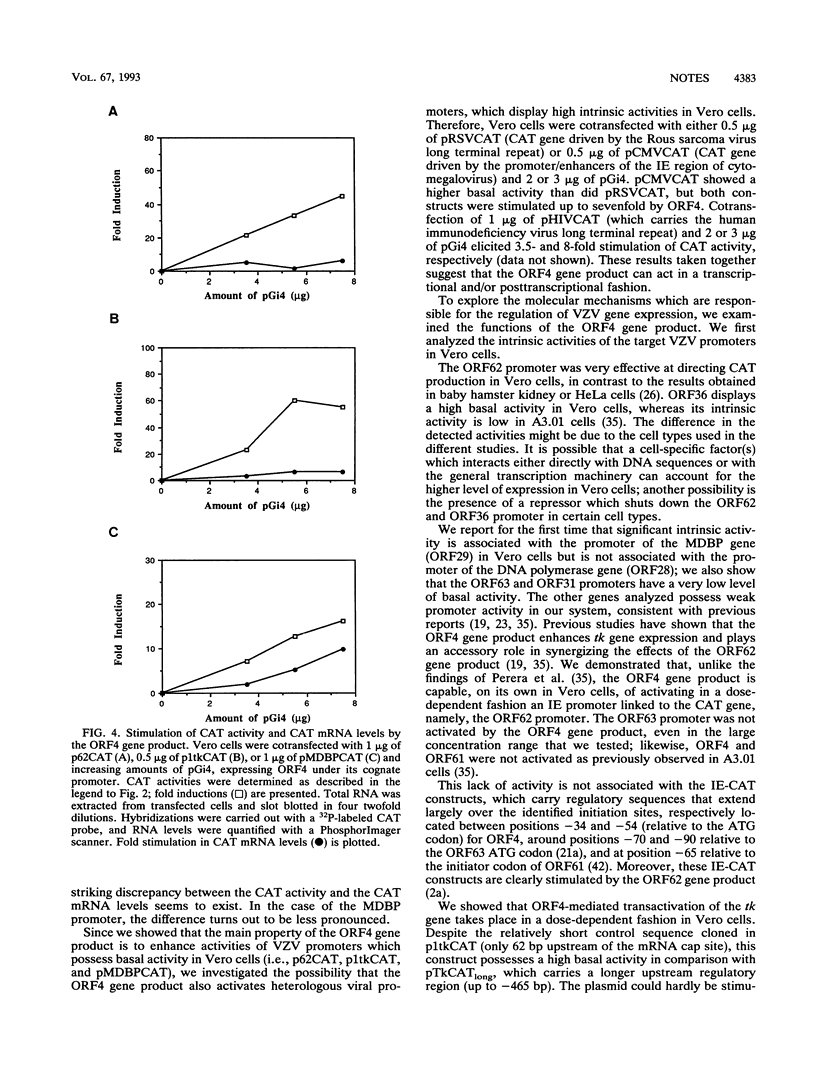
Abstract
Varicella-zoster virus (VZV) open reading frame 4 (ORF4) encodes a protein with a predicted molecular weight of 51,540 presenting amino acid sequence homology with the immediate-early regulatory protein ICP27 of herpes simplex virus type 1. To investigate the regulatory properties of the ORF4 gene product, we performed a series of transient expression assays in Vero cells, using a plasmid expressing ORF4 as effector and several VZV genes and heterologous genes as targets. The VZV target plasmids contained promoter/regulatory regions from genes belonging to the three putative VZV kinetic classes fused to the chloramphenicol acetyltransferase (CAT) gene. The heterologous target plasmids consisted of promoter/regulatory regions of human cytomegalovirus, Rous sarcoma virus, and human immunodeficiency virus type 1 fused to the reporter gene. These experiments demonstrated that the ORF4 gene product activated expression of ORF62 in a dose-dependent fashion but had no effect on the expression of the three other putative immediate-early genes (ORF4, ORF61, and ORF63). When various amounts of ORF4 were transfected in the presence of early gene promoters, dose-dependent transactivation was evidenced with the thymidine kinase gene (ORF36) and the major DNA-binding protein gene (ORF29) promoters; interestingly, little activity was detected with the promoter of the DNA polymerase gene (ORF28). No activation of late gene expression, represented by the glycoprotein I and glycoprotein II genes, was seen even over a wide range of concentrations of input ORF4 plasmid. Expression of pCMVCAT, pRSVCAT, and pHIVCAT was also stimulated by the ORF4 gene product. CAT mRNA analysis showed that activation of VZV target promoters occurs at the transcriptional and/or posttranscriptional level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayer D. E., Dynan W. S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988 May;8(5):2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabirac G. F., Mahalingam R., Wellish M., Gilden D. H. Trans-activation of viral tk promoters by proteins encoded by varicella zoster virus open reading frames 61 and 62. Virus Res. 1990 Jan;15(1):57–68. doi: 10.1016/0168-1702(90)90013-2. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Chapman C. J., Harris J. D., Hardwicke M. A., Sandri-Goldin R. M., Collins M. K., Latchman D. S. Promoter-independent activation of heterologous virus gene expression by the herpes simplex virus immediate-early protein ICP27. Virology. 1992 Feb;186(2):573–578. doi: 10.1016/0042-6822(92)90023-i. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J. Varicella-zoster virus. The Fourteenth Fleming lecture. J Gen Virol. 1991 Mar;72(Pt 3):475–486. doi: 10.1099/0022-1317-72-3-475. [DOI] [PubMed] [Google Scholar]
- Disney G. H., Everett R. D. A herpes simplex virus type 1 recombinant with both copies of the Vmw175 coding sequences replaced by the homologous varicella-zoster virus open reading frame. J Gen Virol. 1990 Nov;71(Pt 11):2681–2689. doi: 10.1099/0022-1317-71-11-2681. [DOI] [PubMed] [Google Scholar]
- Disney G. H., McKee T. A., Preston C. M., Everett R. D. The product of varicella-zoster virus gene 62 autoregulates its own promoter. J Gen Virol. 1990 Dec;71(Pt 12):2999–3003. doi: 10.1099/0022-1317-71-12-2999. [DOI] [PubMed] [Google Scholar]
- Felser J. M., Kinchington P. R., Inchauspe G., Straus S. E., Ostrove J. M. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the "IE" 175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988 Jun;62(6):2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Straus S. E., Ostrove J. M. Varicella-zoster virus complements herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1987 Jan;61(1):225–228. doi: 10.1128/jvi.61.1.225-228.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwicke M. A., Vaughan P. J., Sekulovich R. E., O'Conner R., Sandri-Goldin R. M. The regions important for the activator and repressor functions of herpes simplex virus type 1 alpha protein ICP27 map to the C-terminal half of the molecule. J Virol. 1989 Nov;63(11):4590–4602. doi: 10.1128/jvi.63.11.4590-4602.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe G., Nagpal S., Ostrove J. M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989 Dec;173(2):700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Inchauspe G., Ostrove J. M. Differential regulation by varicella-zoster virus (VZV) and herpes simplex virus type-1 trans-activating genes. Virology. 1989 Dec;173(2):710–714. doi: 10.1016/0042-6822(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Jackers P., Defechereux P., Baudoux L., Lambert C., Massaer M., Merville-Louis M. P., Rentier B., Piette J. Characterization of regulatory functions of the varicella-zoster virus gene 63-encoded protein. J Virol. 1992 Jun;66(6):3899–3903. doi: 10.1128/jvi.66.6.3899-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington P. R., Hougland J. K., Arvin A. M., Ruyechan W. T., Hay J. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J Virol. 1992 Jan;66(1):359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P., Kinchington P. R., Sadeghi-Zadeh M., Ruyechan W. T., Hay J. Transcription from varicella-zoster virus gene 67 (glycoprotein IV). J Virol. 1992 Jun;66(6):3690–3698. doi: 10.1128/jvi.66.6.3690-3698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Structural features of the herpes simplex virus alpha gene 4, 0, and 27 promoter-regulatory sequences which confer alpha regulation on chimeric thymidine kinase genes. J Virol. 1982 Dec;44(3):939–949. doi: 10.1128/jvi.44.3.939-949.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee T. A., Disney G. H., Everett R. D., Preston C. M. Control of expression of the varicella-zoster virus major immediate early gene. J Gen Virol. 1990 Apr;71(Pt 4):897–906. doi: 10.1099/0022-1317-71-4-897. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Phelan A., Loney C., Sandri-Goldin R. M., Clements J. B. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3' processing. J Virol. 1992 Dec;66(12):6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan L., Schaffer P. A. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J Virol. 1990 Jul;64(7):3471–3485. doi: 10.1128/jvi.64.7.3471-3485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Smith H. A., Straus S. E., Cohen J. I. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992 Dec;66(12):7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S., Ostrove J. M. Characterization of a potent varicella-zoster virus-encoded trans-repressor. J Virol. 1991 Oct;65(10):5289–5296. doi: 10.1128/jvi.65.10.5289-5296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M. Molecular biology of varicella zoster virus. Adv Virus Res. 1990;38:45–98. doi: 10.1016/s0065-3527(08)60859-3. [DOI] [PubMed] [Google Scholar]
- Pederson N. E., Person S., Homa F. L. Analysis of the gB promoter of herpes simplex virus type 1: high-level expression requires both an 89-base-pair promoter fragment and a nontranslated leader sequence. J Virol. 1992 Oct;66(10):6226–6232. doi: 10.1128/jvi.66.10.6226-6232.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera L. P., Mosca J. D., Ruyechan W. T., Hay J. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J Virol. 1992 Sep;66(9):5298–5304. doi: 10.1128/jvi.66.9.5298-5304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Mendoza G. E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992 May;6(5):848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- Sekulovich R. E., Leary K., Sandri-Goldin R. M. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988 Dec;62(12):4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K., Hyman R. W. The immediate early proteins of varicella-zoster virus. Virology. 1987 Feb;156(2):423–426. doi: 10.1016/0042-6822(87)90423-5. [DOI] [PubMed] [Google Scholar]
- Smith I. L., Hardwicke M. A., Sandri-Goldin R. M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992 Jan;186(1):74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Stevenson D., Colman K. L., Davison A. J. Characterization of the varicella-zoster virus gene 61 protein. J Gen Virol. 1992 Mar;73(Pt 3):521–530. doi: 10.1099/0022-1317-73-3-521. [DOI] [PubMed] [Google Scholar]
- Su L., Knipe D. M. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology. 1989 Jun;170(2):496–504. doi: 10.1016/0042-6822(89)90441-8. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J., Spector D. J. Heterologous transactivation among viruses. Prog Med Virol. 1989;36:120–190. [PubMed] [Google Scholar]
- Vaughan P. J., Thibault K. J., Hardwicke M. A., Sandri-Goldin R. M. The herpes simplex virus immediate early protein ICP27 encodes a potential metal binding domain and binds zinc in vitro. Virology. 1992 Jul;189(1):377–384. doi: 10.1016/0042-6822(92)90720-a. [DOI] [PubMed] [Google Scholar]
- Wu C. L., Wilcox K. W. The conserved DNA-binding domains encoded by the herpes simplex virus type 1 ICP4, pseudorabies virus IE180, and varicella-zoster virus ORF62 genes recognize similar sites in the corresponding promoters. J Virol. 1991 Mar;65(3):1149–1159. doi: 10.1128/jvi.65.3.1149-1159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


