Abstract
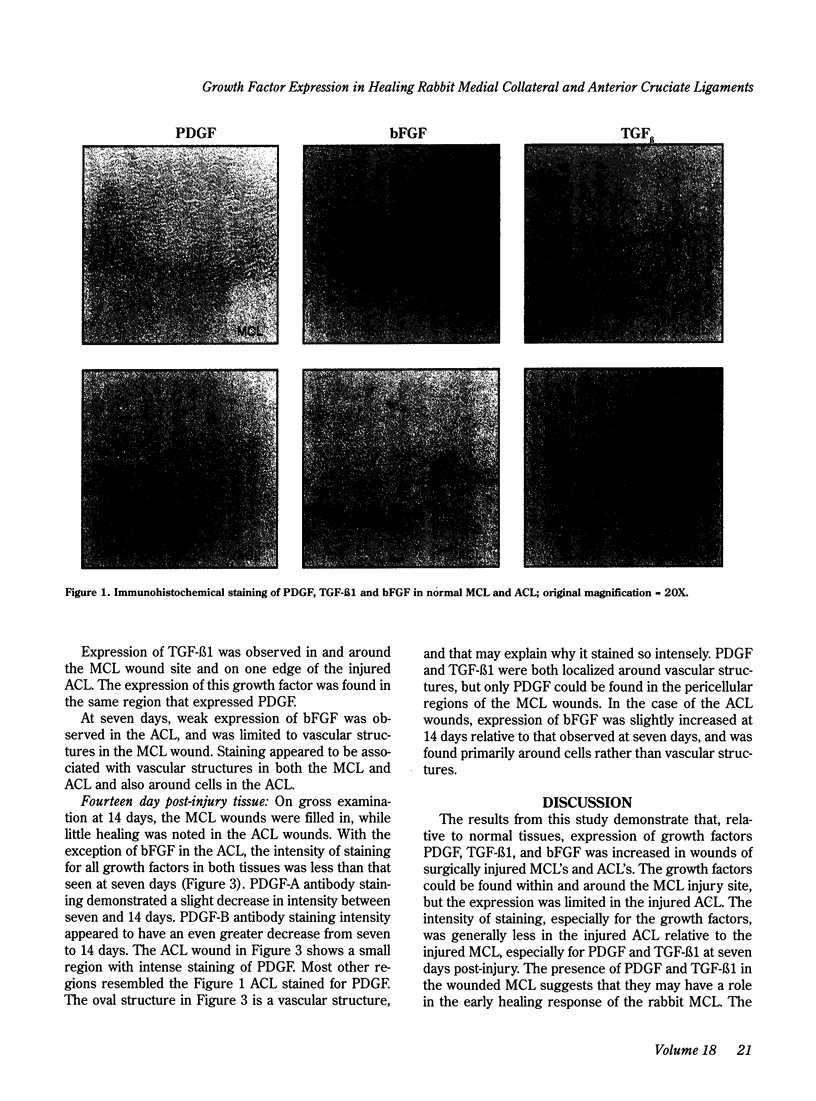
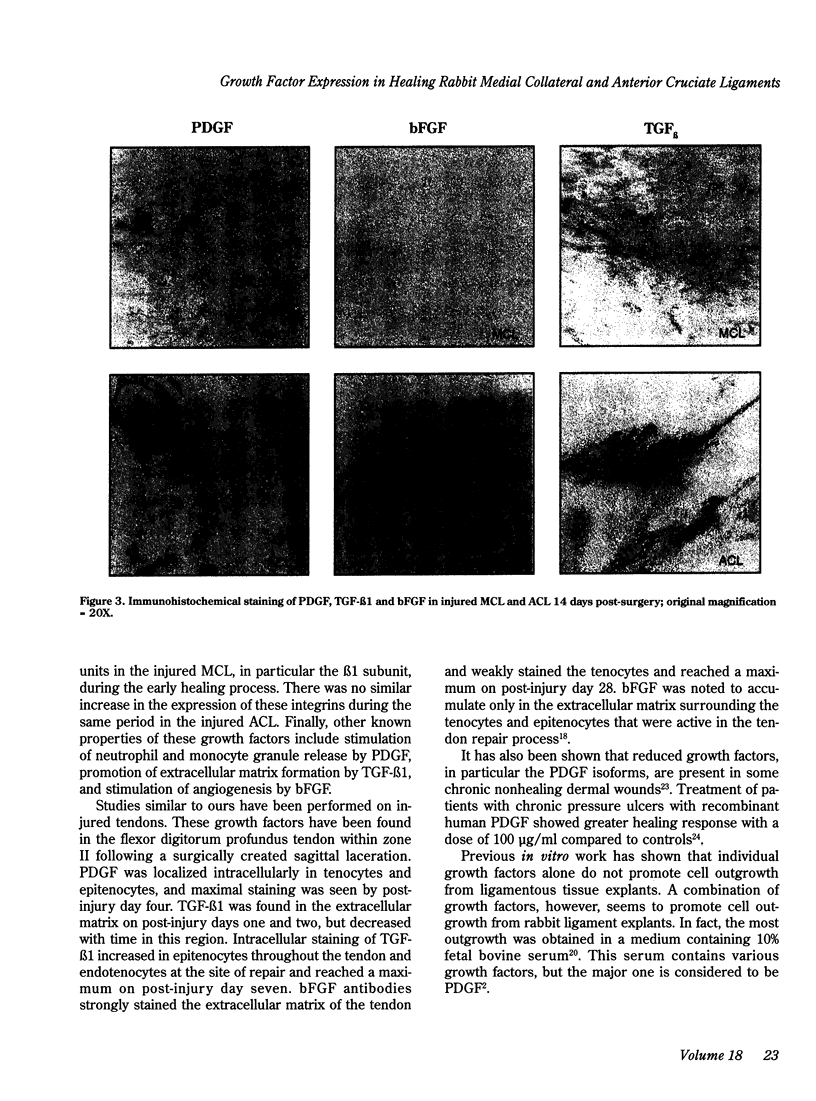
Exogenously administered growth factors such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-beta) and basic fibroblast growth factor (bFGF) have been shown to affect connective tissue healing in vivo, but their intrinsic role in the healing response has not been established. In the present study, immunohistochemistry with antibodies directed against these growth factors showed that expression of PDGF, TGF-beta 1 and bFGF was increased in and around the wound site in the rabbit medial collateral ligament (MCL) seven days following surgical injury. The strong expression of PDGF correlated with the observed increased cellularity consistent with this growth factor's mitogenic and chemotactic properties. Expression of these growth factors was also increased in wounded rabbit anterior cruciate ligaments (ACL) at seven days following surgical injury, but such expression was limited to the edge of the ACL injury site and was of lesser intensity relative to the MCL. This study suggests that PDGF and TGF-beta 1, and to a lesser extent bFGF, are actively involved during the early stage of MCL healing, but have a more limited presence in the injured rabbit ACL.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansel J. C., Tiesman J. P., Olerud J. E., Krueger J. G., Krane J. F., Tara D. C., Shipley G. D., Gilbertson D., Usui M. L., Hart C. E. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J Clin Invest. 1993 Aug;92(2):671–678. doi: 10.1172/JCI116636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Batten M. L., Hansen J. C., Dahners L. E. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res. 1996 Sep;14(5):736–741. doi: 10.1002/jor.1100140509. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Kim J. P., Zhang K., Sarret Y., Wynn K. C., Kramer R. H., Woodley D. T. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993 Dec;209(2):216–223. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Vlodavsky I., Haimovitz-Friedman A., Hicklin D., Fuks Z. Expression of basic fibroblast growth factor in normal human tissues. Lab Invest. 1990 Dec;63(6):832–840. [PubMed] [Google Scholar]
- Cromack D. T., Sporn M. B., Roberts A. B., Merino M. J., Dart L. L., Norton J. A. Transforming growth factor beta levels in rat wound chambers. J Surg Res. 1987 Jun;42(6):622–628. doi: 10.1016/0022-4804(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Enenstein J., Waleh N. S., Kramer R. H. Basic FGF and TGF-beta differentially modulate integrin expression of human microvascular endothelial cells. Exp Cell Res. 1992 Dec;203(2):499–503. doi: 10.1016/0014-4827(92)90028-7. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor: structural and biological properties. J Cell Physiol Suppl. 1987;Suppl 5:15–26. doi: 10.1002/jcp.1041330405. [DOI] [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Janat M. F., Argraves W. S., Liau G. Regulation of vascular smooth muscle cell integrin expression by transforming growth factor beta1 and by platelet-derived growth factor-BB. J Cell Physiol. 1992 Jun;151(3):588–595. doi: 10.1002/jcp.1041510319. [DOI] [PubMed] [Google Scholar]
- Joyce M. E., Roberts A. B., Sporn M. B., Bolander M. E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990 Jun;110(6):2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Green M. H., Amiel D. Synergistic effect of growth factors on cell outgrowth from explants of rabbit anterior cruciate and medial collateral ligaments. J Orthop Res. 1995 May;13(3):435–441. doi: 10.1002/jor.1100130318. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Colvin R. B., Antoniades H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989 Aug;84(2):640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Tarpley J. E., Tseng J., Bready J., Chang D., Kenney W. C., Rudolph R., Robson M. C., Vande Berg J., Reid P. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995 Sep;96(3):1336–1350. doi: 10.1172/JCI118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson M. C., Phillips L. G., Thomason A., Robson L. E., Pierce G. F. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet. 1992 Jan 4;339(8784):23–25. doi: 10.1016/0140-6736(92)90143-q. [DOI] [PubMed] [Google Scholar]
- Schreck P. J., Kitabayashi L. R., Amiel D., Akeson W. H., Woods V. L., Jr Integrin display increases in the wounded rabbit medial collateral ligament but not the wounded anterior cruciate ligament. J Orthop Res. 1995 Mar;13(2):174–183. doi: 10.1002/jor.1100130205. [DOI] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Siegbahn A., Hammacher A., Westermark B., Heldin C. H. Differential effects of the various isoforms of platelet-derived growth factor on chemotaxis of fibroblasts, monocytes, and granulocytes. J Clin Invest. 1990 Mar;85(3):916–920. doi: 10.1172/JCI114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Baehner R. L. Platelet-derived growth factor promotes human peripheral monocyte activation. Blood. 1985 Jul;66(1):179–183. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Allen J. B., Dougherty E. B., Dougherty S. F. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- Wallace C. D., Amiel D. Vascular assessment of the periarticular ligaments of the rabbit knee. J Orthop Res. 1991 Nov;9(6):787–791. doi: 10.1002/jor.1100090603. [DOI] [PubMed] [Google Scholar]
- Wiig M. E., Amiel D., Ivarsson M., Nagineni C. N., Wallace C. D., Arfors K. E. Type I procollagen gene expression in normal and early healing of the medial collateral and anterior cruciate ligaments in rabbits: an in situ hybridization study. J Orthop Res. 1991 May;9(3):374–382. doi: 10.1002/jor.1100090309. [DOI] [PubMed] [Google Scholar]
- Wiig M. E., Amiel D., VandeBerg J., Kitabayashi L., Harwood F. L., Arfors K. E. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: an experimental study in rabbits. J Orthop Res. 1990 May;8(3):425–434. doi: 10.1002/jor.1100080314. [DOI] [PubMed] [Google Scholar]