Abstract
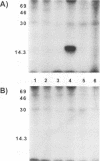
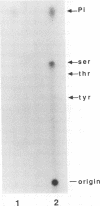
Nucleotide sequencing of the SalI j region of the virulent Malawi (LIL20/1) strain of African swine fever virus (ASFV) identified an open reading frame (ORF), designated j9L, with extensive similarity to the family of protein kinases. This ORF encodes a 35.1-kDa protein of 299 amino acids which shares 24.6% amino acid identity with the human pim-1 proto-oncogene and 21.0% identity with the vaccinia virus B1R-encoded protein kinase. The ASFV ORF contains the motifs characteristic of serine-threonine protein kinases, with the exception of the presumed ATP-binding site, which is poorly conserved. The ORF was expressed to high levels in Escherichia coli, and the recombinant enzyme phosphorylated a calf thymus histone protein on serine residues in vitro. An antibody raised to an amino-terminal peptide of the ASFV protein kinase was reactive with the recombinant protein in Western immunoblot analyses and was used to demonstrate the presence of the protein kinase in ASF virions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcamí A., Angulo A., López-Otín C., Muñoz M., Freije J. M., Carrascosa A. L., Viñuela E. Amino acid sequence and structural properties of protein p12, an African swine fever virus attachment protein. J Virol. 1992 Jun;66(6):3860–3868. doi: 10.1128/jvi.66.6.3860-3868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazán F., Rodríguez J. M., Andrés G., Pérez R., Viñuela E., Rodriguez J. F. Transcriptional analysis of multigene family 110 of African swine fever virus. J Virol. 1992 Nov;66(11):6655–6667. doi: 10.1128/jvi.66.11.6655-6667.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendral J. M., Almazán F., Blasco R., Viñuela E. Multigene families in African swine fever virus: family 110. J Virol. 1990 May;64(5):2064–2072. doi: 10.1128/jvi.64.5.2064-2072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banham A. H., Smith G. L. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992 Dec;191(2):803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Barros M. F., Cunha C. V., Costa J. V. Single-stranded deoxyribonucleic acid nuclease induced by African swine fever virus and associated to the virion. Virology. 1986 Nov;155(1):183–191. doi: 10.1016/0042-6822(86)90178-9. [DOI] [PubMed] [Google Scholar]
- Baylis S. A., Dixon L. K., Vydelingum S., Smith G. L. African swine fever virus encodes a gene with extensive homology to type II DNA topoisomerases. J Mol Biol. 1992 Dec 5;228(3):1003–1010. doi: 10.1016/0022-2836(92)90887-p. [DOI] [PubMed] [Google Scholar]
- Blasco R., López-Otín C., Muñz M., Bockamp E. O., Simón-Mateo C., Viñuela E. Sequence and evolutionary relationships of African swine fever virus thymidine kinase. Virology. 1990 Sep;178(1):301–304. doi: 10.1016/0042-6822(90)90409-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M., Shaw K., Yáez R. J., Viñuela E., Dixon L. The sequences of the ribonucleotide reductase genes from African swine fever virus show considerable homology with those of the orthopoxvirus, vaccinia virus. Virology. 1991 Sep;184(1):411–416. doi: 10.1016/0042-6822(91)90860-e. [DOI] [PubMed] [Google Scholar]
- Camacho A., Viñuela E. Protein p22 of African swine fever virus: an early structural protein that is incorporated into the membrane of infected cells. Virology. 1991 Mar;181(1):251–257. doi: 10.1016/0042-6822(91)90490-3. [DOI] [PubMed] [Google Scholar]
- Carrascosa A. L., del Val M., Santarén J. F., Viñuela E. Purification and properties of African swine fever virus. J Virol. 1985 May;54(2):337–344. doi: 10.1128/jvi.54.2.337-344.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Lawrence G. L., Barrell B. G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989 May;70(Pt 5):1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
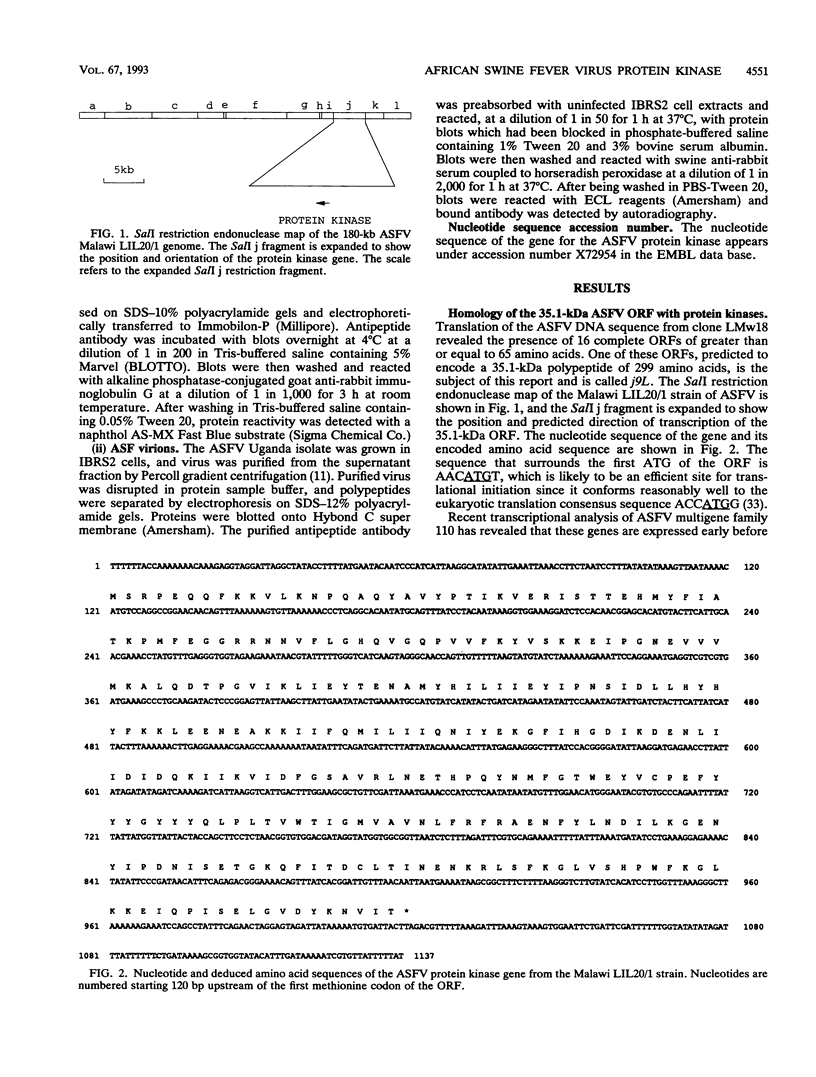
- Dixon L. K. Molecular cloning and restriction enzyme mapping of an African swine fever virus isolate from Malawi. J Gen Virol. 1988 Jul;69(Pt 7):1683–1694. doi: 10.1099/0022-1317-69-7-1683. [DOI] [PubMed] [Google Scholar]
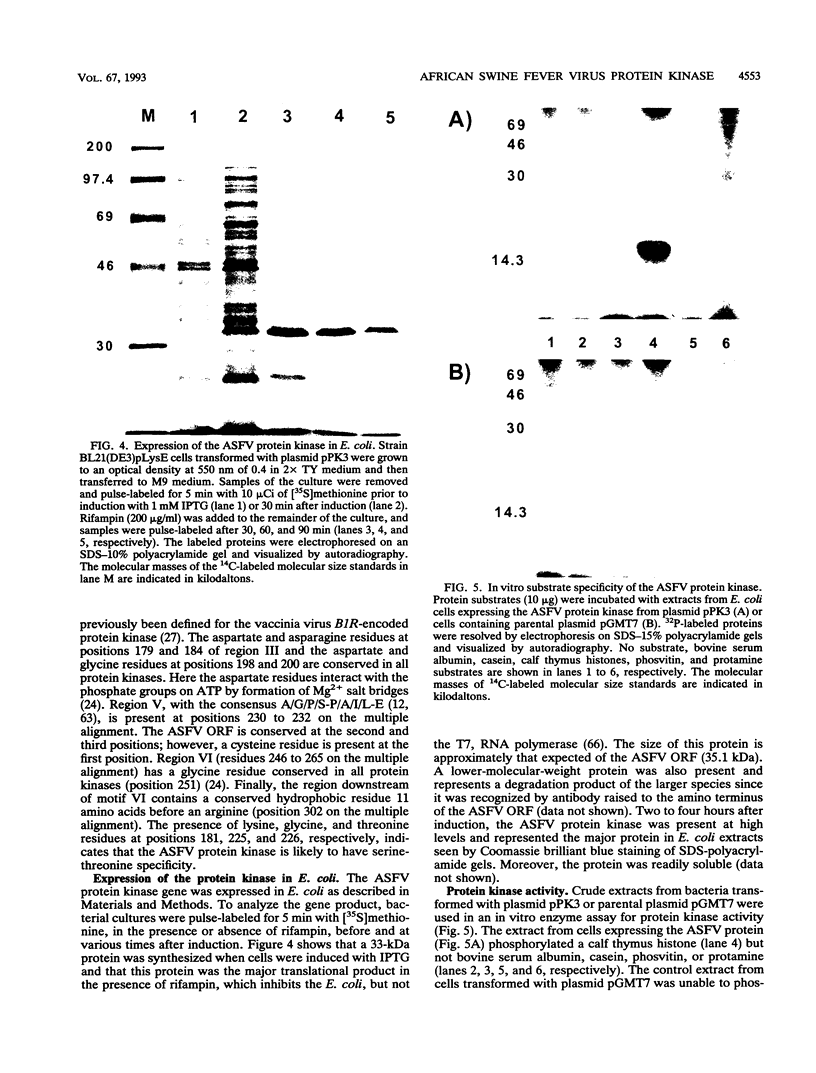
- Esteves A., Marques M. I., Costa J. V. Two-dimensional analysis of African swine fever virus proteins and proteins induced in infected cells. Virology. 1986 Jul 15;152(1):192–206. doi: 10.1016/0042-6822(86)90384-3. [DOI] [PubMed] [Google Scholar]
- García-Beato R., Freije J. M., López-Otín C., Blasco R., Viñuela E., Salas M. L. A gene homologous to topoisomerase II in African swine fever virus. Virology. 1992 Jun;188(2):938–947. doi: 10.1016/0042-6822(92)90558-7. [DOI] [PubMed] [Google Scholar]
- García-Beato R., Salas M. L., Viñuela E., Salas J. Role of the host cell nucleus in the replication of African swine fever virus DNA. Virology. 1992 Jun;188(2):637–649. doi: 10.1016/0042-6822(92)90518-t. [DOI] [PubMed] [Google Scholar]
- González A., Calvo V., Almazán F., Almendral J. M., Ramírez J. C., de la Vega I., Blasco R., Viñuela E. Multigene families in African swine fever virus: family 360. J Virol. 1990 May;64(5):2073–2081. doi: 10.1128/jvi.64.5.2073-2081.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
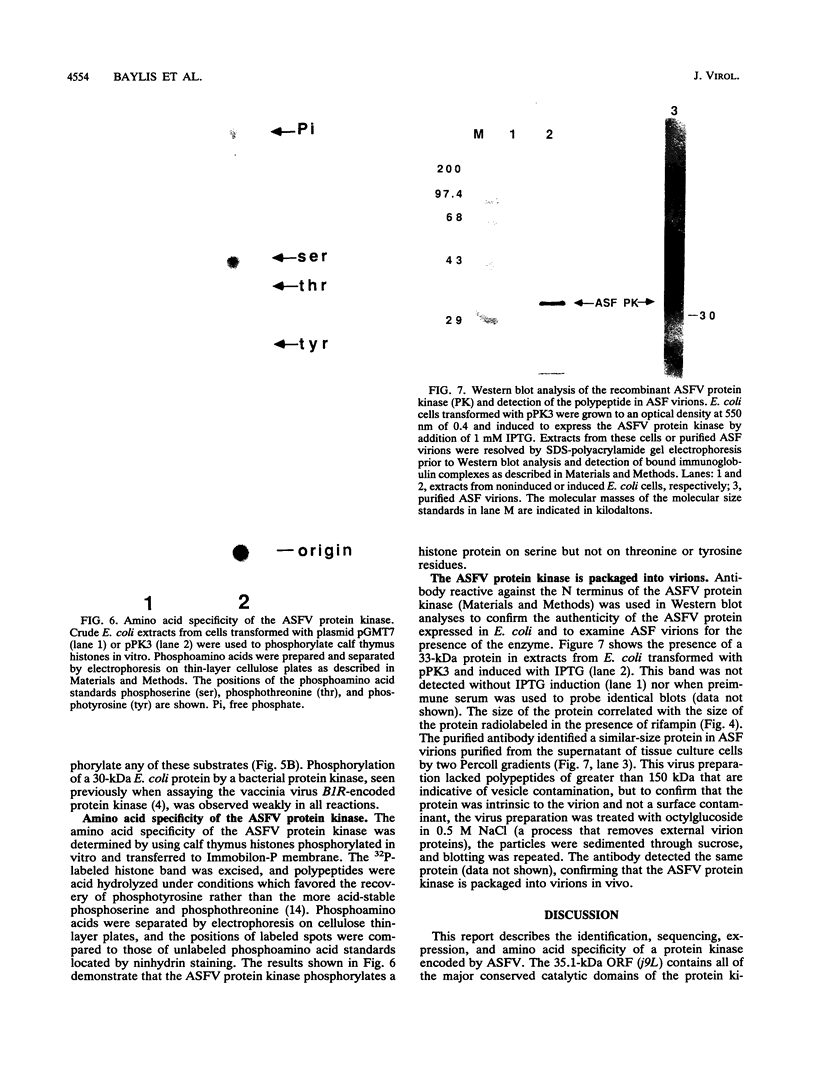
- Hammond J. M., Kerr S. M., Smith G. L., Dixon L. K. An African swine fever virus gene with homology to DNA ligases. Nucleic Acids Res. 1992 Jun 11;20(11):2667–2671. doi: 10.1093/nar/20.11.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hingamp P. M., Arnold J. E., Mayer R. J., Dixon L. K. A ubiquitin conjugating enzyme encoded by African swine fever virus. EMBO J. 1992 Jan;11(1):361–366. doi: 10.1002/j.1460-2075.1992.tb05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. T., Smith G. L. Two early vaccinia virus genes encode polypeptides related to protein kinases. J Gen Virol. 1989 Dec;70(Pt 12):3187–3201. doi: 10.1099/0022-1317-70-12-3187. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Characterization of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2430–2437. [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Purification of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2420–2429. [PubMed] [Google Scholar]
- Kleiman J., Moss B. Protein kinase activity from vaccinia virions: solubilization and separation into heat-labile and heat-stable components. J Virol. 1973 Oct;12(4):684–689. doi: 10.1128/jvi.12.4.684-689.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kuznar J., Salas M. L., Viñuela E. DNA-dependent RNA polymerase in African swine fever virus. Virology. 1980 Feb;101(1):169–175. doi: 10.1016/0042-6822(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Kuznar J., Salas M. L., Viñuela E. Nucleoside triphosphate phosphohydrolase activities in African swine fever virus. Arch Virol. 1981;69(3-4):307–310. doi: 10.1007/BF01317347. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leader D. P. Identification of protein kinases by computer. Nature. 1988 May 26;333(6171):308–308. doi: 10.1038/333308a0. [DOI] [PubMed] [Google Scholar]
- Lin S., Chen W., Broyles S. S. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J Virol. 1992 May;66(5):2717–2723. doi: 10.1128/jvi.66.5.2717-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991 Mar 22;64(6):1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- López-Otín C., Freije J. M., Parra F., Méndez E., Viñuela E. Mapping and sequence of the gene coding for protein p72, the major capsid protein of African swine fever virus. Virology. 1990 Apr;175(2):477–484. doi: 10.1016/0042-6822(90)90432-q. [DOI] [PubMed] [Google Scholar]
- López-Otín C., Simón C., Méndez E., Viñuela E. Mapping and sequence of the gene encoding protein p37, a major structural protein of African swine fever virus. Virus Genes. 1988 Jun;1(3):291–303. doi: 10.1007/BF00572708. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Jinno A., Takagi N., Shibuya M. A novel mammalian protein kinase gene (mak) is highly expressed in testicular germ cells at and after meiosis. Mol Cell Biol. 1990 May;10(5):2261–2268. doi: 10.1128/mcb.10.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus C. A. African swine fever. Adv Virus Res. 1988;35:251–269. doi: 10.1016/s0065-3527(08)60714-9. [DOI] [PubMed] [Google Scholar]
- Ortin J., Vińuela E. Requirement of cell nucleus for African swine fever virus replication in Vero cells. J Virol. 1977 Mar;21(3):902–905. doi: 10.1128/jvi.21.3.902-905.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polatnick J., Pan I. C., Gravell M. Protein kinase activity in African swine fever virus. Arch Gesamte Virusforsch. 1974;44(2):156–159. doi: 10.1007/BF01250227. [DOI] [PubMed] [Google Scholar]
- Reeves R., Spies G. A., Kiefer M., Barr P. J., Power M. Primary structure of the putative human oncogene, pim-1. Gene. 1990 Jun 15;90(2):303–307. doi: 10.1016/0378-1119(90)90195-w. [DOI] [PubMed] [Google Scholar]
- Rempel R. E., Anderson M. K., Evans E., Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990 Feb;64(2):574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel R. E., Traktman P. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J Virol. 1992 Jul;66(7):4413–4426. doi: 10.1128/jvi.66.7.4413-4426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J. M., Almazán F., Viñuela E., Rodriguez J. F. Genetic manipulation of African swine fever virus: construction of recombinant viruses expressing the beta-galactosidase gene. Virology. 1992 May;188(1):67–76. doi: 10.1016/0042-6822(92)90735-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987 May 22;49(4):569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Salas M. L., Kuznar J., Viñuela E. Effect of rifamycin derivatives and coumermycin A1 on in vitro RNA synthesis by African swine fever virus. Brief report. Arch Virol. 1983;77(1):77–80. doi: 10.1007/BF01314866. [DOI] [PubMed] [Google Scholar]
- Salas M. L., Kuznar J., Viñuela E. Polyadenylation, methylation, and capping of the RNA synthesized in vitro by African swine fever virus. Virology. 1981 Sep;113(2):484–491. doi: 10.1016/0042-6822(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Salas M. L., Salas J., Viñuela E. Phosphorylation of African swine fever virus proteins in vitro and in vivo. Biochimie. 1988 May;70(5):627–635. doi: 10.1016/0300-9084(88)90246-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarén J. F., Viñuela E. African swine fever virus-induced polypeptides in Vero cells. Virus Res. 1986 Sep;5(4):391–405. doi: 10.1016/0168-1702(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Saxena A., Padmanabha R., Glover C. V. Isolation and sequencing of cDNA clones encoding alpha and beta subunits of Drosophila melanogaster casein kinase II. Mol Cell Biol. 1987 Oct;7(10):3409–3417. doi: 10.1128/mcb.7.10.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Boelens W., Robanus-Maandag E., Verbeek J., Domen J., van Beveren C., Berns A. The primary structure of the putative oncogene pim-1 shows extensive homology with protein kinases. Cell. 1986 Aug 15;46(4):603–611. doi: 10.1016/0092-8674(86)90886-x. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989 Jan;63(1):450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An improved sequence handling package that runs on the Apple Macintosh. Comput Appl Biosci. 1990 Oct;6(4):387–393. doi: 10.1093/bioinformatics/6.4.387. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Traktman P., Anderson M. K., Rempel R. E. Vaccinia virus encodes an essential gene with strong homology to protein kinases. J Biol Chem. 1989 Dec 25;264(36):21458–21461. [PubMed] [Google Scholar]
- Urzainqui A., Tabarés E., Carrasco L. Proteins synthesized in African swine fever virus-infected cells analyzed by two-dimensional gel electrophoresis. Virology. 1987 Sep;160(1):286–291. doi: 10.1016/0042-6822(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Valdeira M. L., Duque-Magalhães M. C., Geraldes A. Evidence for an acid phosphatase in African swine fever virus. Arch Virol. 1990;113(1-2):125–131. doi: 10.1007/BF01318361. [DOI] [PubMed] [Google Scholar]
- Viñuela E. African swine fever virus. Curr Top Microbiol Immunol. 1985;116:151–170. doi: 10.1007/978-3-642-70280-8_8. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990 Aug;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]