Abstract
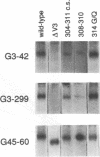
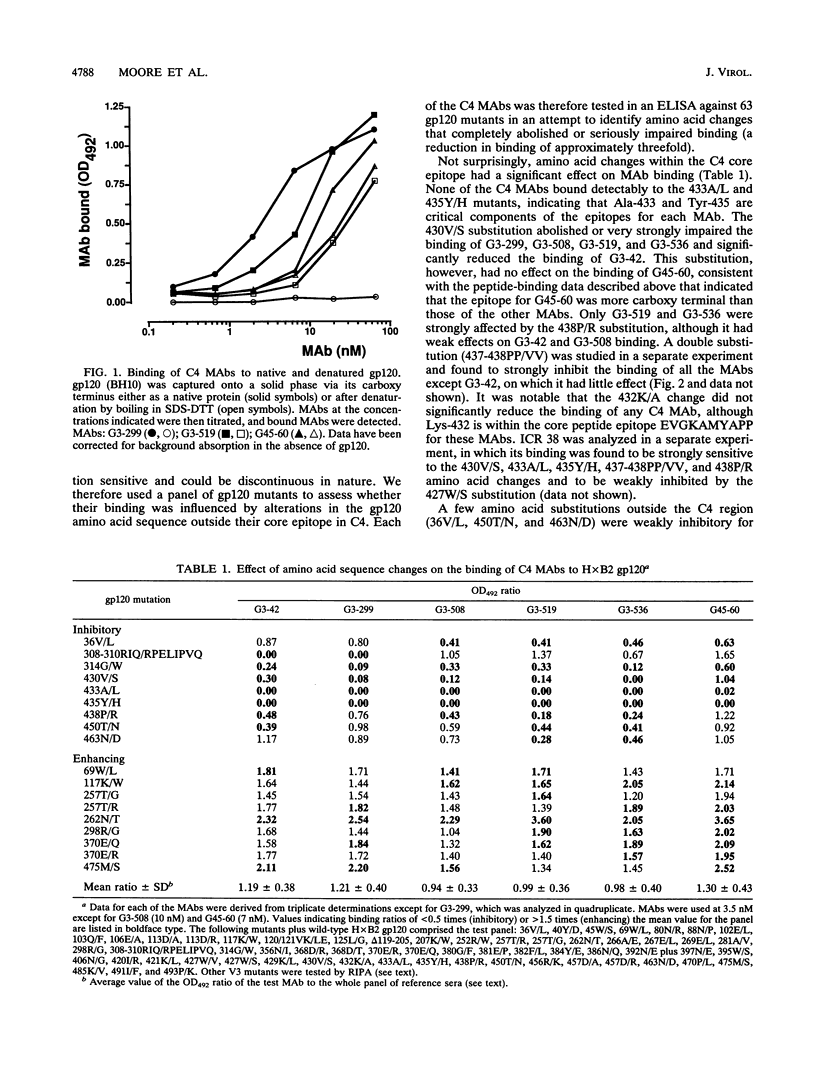
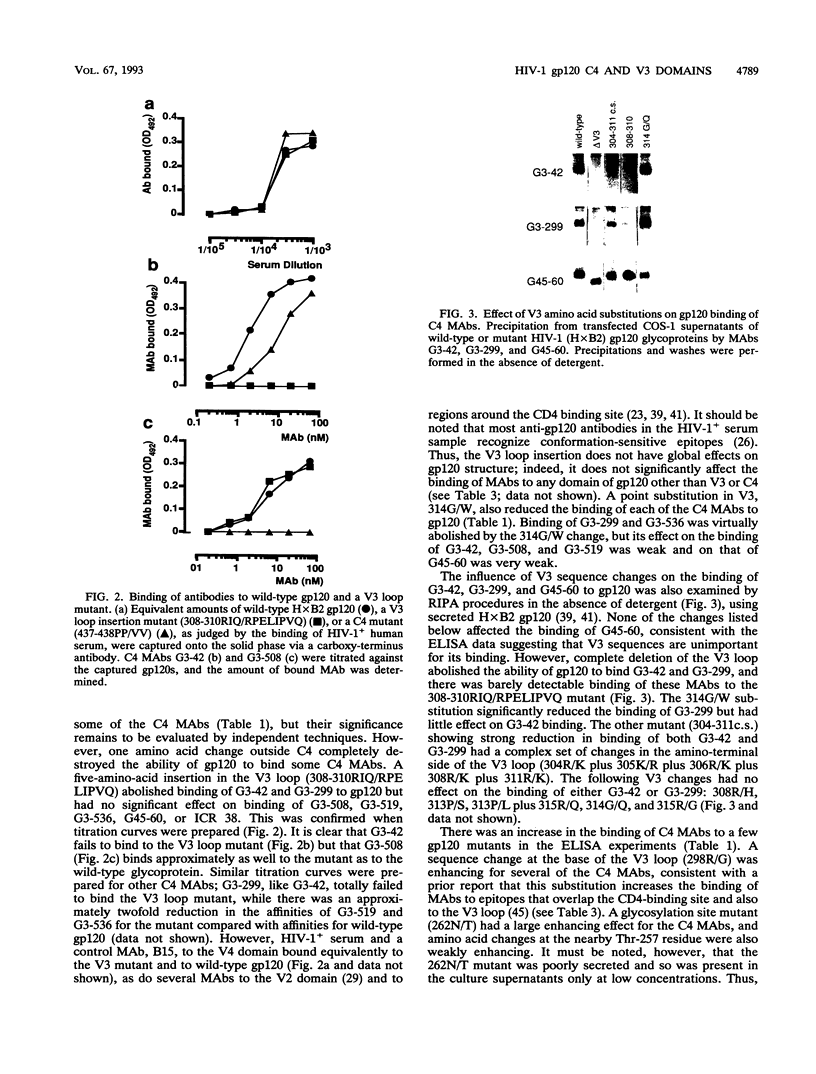
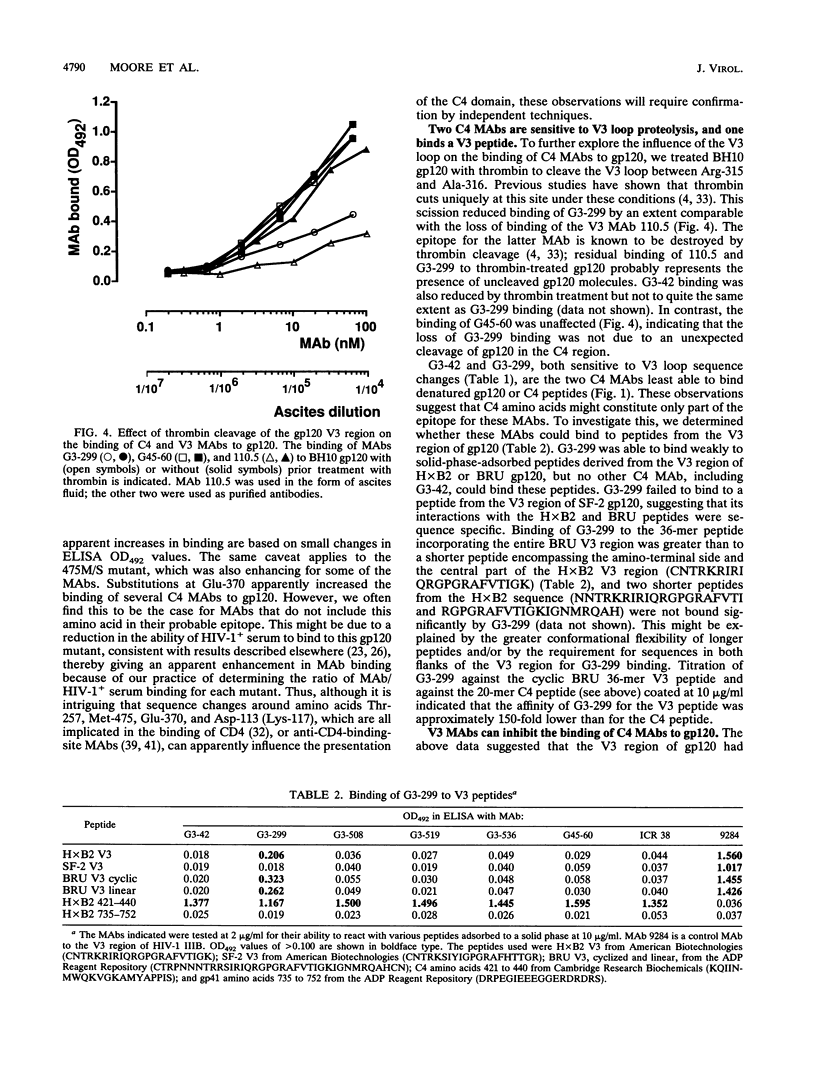
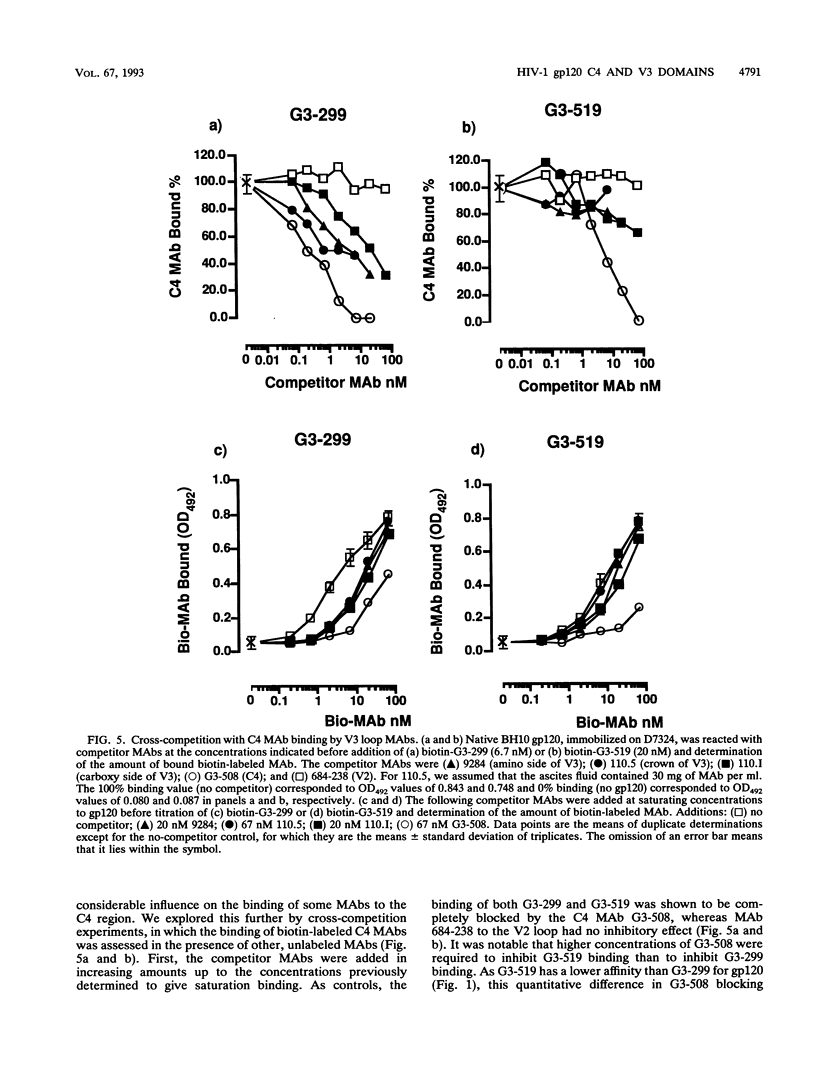
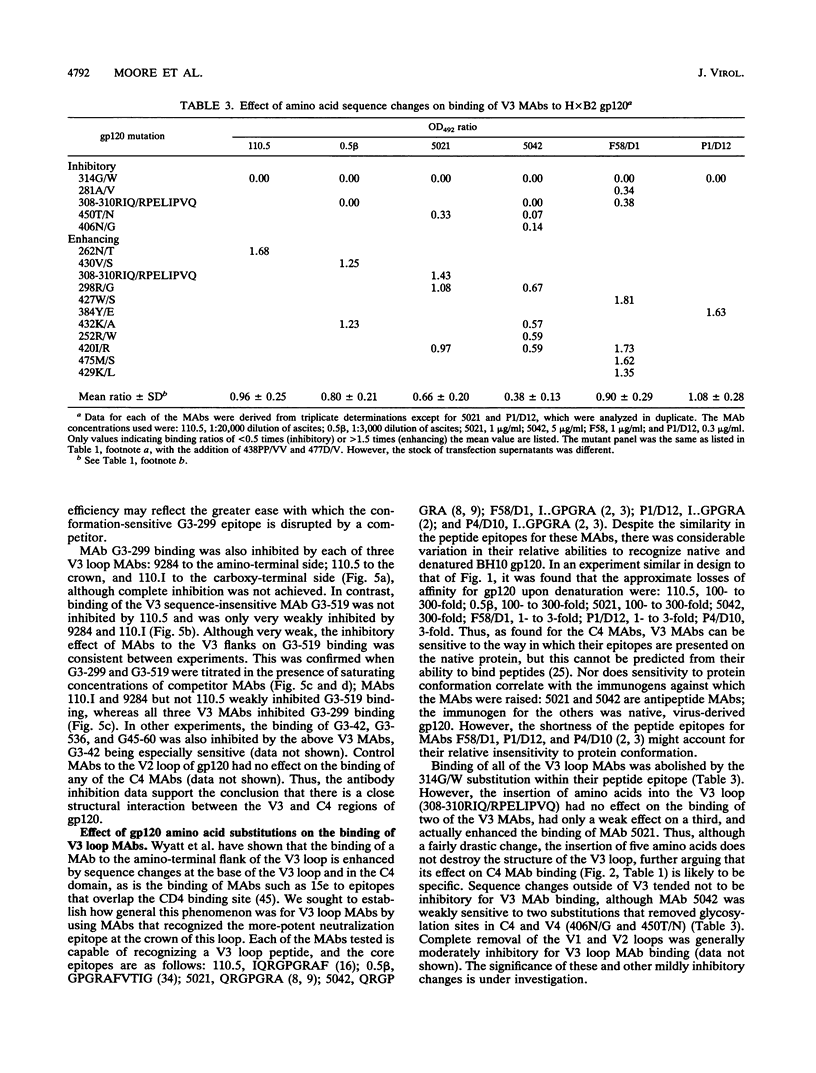
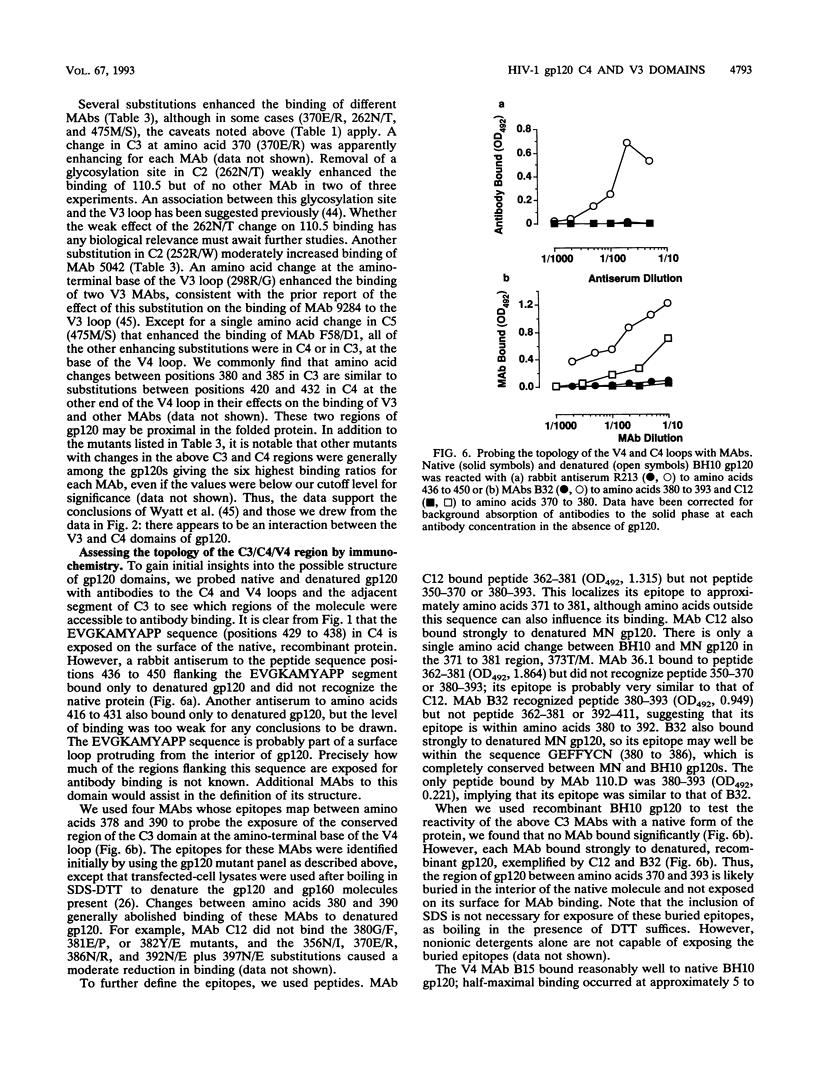
We have probed the structure of the C4 and V3 domains of human immunodeficiency virus type 1 gp120 by immunochemical techniques. Monoclonal antibodies (MAbs) recognizing an exposed gp120 sequence, (E/K)VGKAMYAPP, in C4 were differentially sensitive to denaturation of gp120, implying a conformational component to some of the epitopes. The MAbs recognizing conformation-sensitive C4 structures failed to bind to a gp120 mutant with an alteration in the sequence of the V3 loop, and their binding to gp120 was inhibited by both V3 and C4 MAbs. This implies an interaction between the V3 and C4 regions of gp120, which is supported by the observation that the binding of some MAbs to the V3 loop was often enhanced by amino acid changes in an around the C4 region.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Hinkula J., Broliden P. A., Mäkitalo B., Fridberger T., Rosen J., Villacres-Eriksson M., Morein B., Wahren B. Neutralizing cross-reactive and non-neutralizing monoclonal antibodies to HIV-1 gp120. AIDS. 1990 Oct;4(10):953–960. doi: 10.1097/00002030-199010000-00002. [DOI] [PubMed] [Google Scholar]
- Broliden P. A., Mäkitalo B., Akerblom L., Rosen J., Broliden K., Utter G., Jondal M., Norrby E., Wahren B. Identification of amino acids in the V3 region of gp120 critical for virus neutralization by human HIV-1-specific antibodies. Immunology. 1991 Aug;73(4):371–376. [PMC free article] [PubMed] [Google Scholar]
- Clements G. J., Price-Jones M. J., Stephens P. E., Sutton C., Schulz T. F., Clapham P. R., McKeating J. A., McClure M. O., Thomson S., Marsh M. The V3 loops of the HIV-1 and HIV-2 surface glycoproteins contain proteolytic cleavage sites: a possible function in viral fusion? AIDS Res Hum Retroviruses. 1991 Jan;7(1):3–16. doi: 10.1089/aid.1991.7.3. [DOI] [PubMed] [Google Scholar]
- Cordell J., Moore J. P., Dean C. J., Klasse P. J., Weiss R. A., McKeating J. A. Rat monoclonal antibodies to nonoverlapping epitopes of human immunodeficiency virus type 1 gp120 block CD4 binding in vitro. Virology. 1991 Nov;185(1):72–79. doi: 10.1016/0042-6822(91)90755-z. [DOI] [PubMed] [Google Scholar]
- Cordonnier A., Montagnier L., Emerman M. Single amino-acid changes in HIV envelope affect viral tropism and receptor binding. Nature. 1989 Aug 17;340(6234):571–574. doi: 10.1038/340571a0. [DOI] [PubMed] [Google Scholar]
- Dowbenko D., Nakamura G., Fennie C., Shimasaki C., Riddle L., Harris R., Gregory T., Lasky L. Epitope mapping of the human immunodeficiency virus type 1 gp120 with monoclonal antibodies. J Virol. 1988 Dec;62(12):4703–4711. doi: 10.1128/jvi.62.12.4703-4711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durda P. J., Bacheler L., Clapham P., Jenoski A. M., Leece B., Matthews T. J., McKnight A., Pomerantz R., Rayner M., Weinhold K. J. HIV-1 neutralizing monoclonal antibodies induced by a synthetic peptide. AIDS Res Hum Retroviruses. 1990 Sep;6(9):1115–1123. doi: 10.1089/aid.1990.6.1115. [DOI] [PubMed] [Google Scholar]
- Durda P. J., Leece B., Jenoski A., Rabin H., Fisher A., Wong-Staal F. Characterization of murine monoclonal antibodies to HIV-1 induced by synthetic peptides. AIDS Res Hum Retroviruses. 1988 Oct;4(5):331–342. doi: 10.1089/aid.1988.4.331. [DOI] [PubMed] [Google Scholar]
- Helseth E., Kowalski M., Gabuzda D., Olshevsky U., Haseltine W., Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990 May;64(5):2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Olshevsky U., Furman C., Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991 Apr;65(4):2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., Schmidt S., Kaufmann M., Cates N., Langedijk J. P., Meloen R. H., Desrosiers R. C., Burns D. P., Bolognesi D. P. The principal neutralization determinant of simian immunodeficiency virus differs from that of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1418–1422. doi: 10.1073/pnas.89.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Langedijk J. P., Back N. K., Kinney-Thomas E., Bruck C., Francotte M., Goudsmit J., Meloen R. H. Comparison and fine mapping of both high and low neutralizing monoclonal antibodies against the principal neutralization domain of HIV-1. Arch Virol. 1992;126(1-4):129–146. doi: 10.1007/BF01309690. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan F. E., Hegerich P. A., Brennan T. P., Phanuphak P., Singharaj P., Jugsudee A., Berman P. W., Gray A. M., Fowler A. K., Burke D. S. Genetic variants of HIV-1 in Thailand. AIDS Res Hum Retroviruses. 1992 Nov;8(11):1887–1895. doi: 10.1089/aid.1992.8.1887. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., Moore J. P., Ferguson M., Marsden H. S., Graham S., Almond J. W., Evans D. J., Weiss R. A. Monoclonal antibodies to the C4 region of human immunodeficiency virus type 1 gp120: use in topological analysis of a CD4 binding site. AIDS Res Hum Retroviruses. 1992 Apr;8(4):451–459. doi: 10.1089/aid.1992.8.451. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., Thali M., Furman C., Karwowska S., Gorny M. K., Cordell J., Zolla-Pazner S., Sodroski J., Weiss R. A. Amino acid residues of the human immunodeficiency virus type I gp120 critical for the binding of rat and human neutralizing antibodies that block the gp120-sCD4 interaction. Virology. 1992 Sep;190(1):134–142. doi: 10.1016/0042-6822(92)91199-5. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Ho D. D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993 Feb;67(2):863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Jones I. M., Stephens P. E., Clements G., Thomson S., Weiss R. A. Characterization of recombinant gp120 and gp160 from HIV-1: binding to monoclonal antibodies and soluble CD4. AIDS. 1990 Apr;4(4):307–315. doi: 10.1097/00002030-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Nara P. L. The role of the V3 loop of gp120 in HIV infection. AIDS. 1991;5 (Suppl 2):S21–S33. doi: 10.1097/00002030-199101001-00004. [DOI] [PubMed] [Google Scholar]
- Moore J. P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbent assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS. 1990 Apr;4(4):297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Moore J. P. The reactivities of HIV-1+ human sera with solid-phase V3 loop peptides can be poor predictors of their reactivities with V3 loops on native gp120 molecules. AIDS Res Hum Retroviruses. 1993 Mar;9(3):209–219. doi: 10.1089/aid.1993.9.209. [DOI] [PubMed] [Google Scholar]
- Nakamura G. R., Byrn R., Rosenthal K., Porter J. P., Hobbs M. R., Riddle L., Eastman D. J., Dowbenko D., Gregory T., Fendly B. M. Monoclonal antibodies to the extracellular domain of HIV-1IIIB gp160 that neutralize infectivity, block binding to CD4, and react with diverse isolates. AIDS Res Hum Retroviruses. 1992 Nov;8(11):1875–1885. doi: 10.1089/aid.1992.8.1875. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Helseth E., Furman C., Li J., Haseltine W., Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J Virol. 1990 Dec;64(12):5701–5707. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Ting R., Langlois A. J., Weinhold K. J., Lyerly H. K., Javaherian K., Matthews T. J. Characteristics of a neutralizing monoclonal antibody to the HIV envelope glycoprotein. AIDS Res Hum Retroviruses. 1988 Jun;4(3):187–197. doi: 10.1089/aid.1988.4.187. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Scandella C. J., Skiles P. V., Haigwood N. L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science. 1991 Oct 4;254(5028):105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- Sullivan N., Thali M., Furman C., Ho D. D., Sodroski J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J Virol. 1993 Jun;67(6):3674–3679. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N. C., Ho D. D., Sun C. R., Liou R. S., Gordon W., Fung M. S., Li X. L., Ting R. C., Lee T. H., Chang N. T. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3579–3585. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Furman C., Helseth E., Repke H., Sodroski J. Lack of correlation between soluble CD4-induced shedding of the human immunodeficiency virus type 1 exterior envelope glycoprotein and subsequent membrane fusion events. J Virol. 1992 Sep;66(9):5516–5524. doi: 10.1128/jvi.66.9.5516-5524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Furman C., Ho D. D., Robinson J., Tilley S., Pinter A., Sodroski J. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 1992 Sep;66(9):5635–5641. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Moore J. P., Furman C., Charles M., Ho D. D., Robinson J., Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993 Jul;67(7):3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Olshevsky U., Furman C., Gabuzda D., Posner M., Sodroski J. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J Virol. 1991 Nov;65(11):6188–6193. doi: 10.1128/jvi.65.11.6188-6193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriart C., Francotte M., Cohen J., Collignon C., Delers A., Kummert S., Molitor C., Gilles D., Roelants P., van Wijnendaele F. Several antigenic determinants exposed on the gp120 moiety of HIV-1 gp160 are hidden on the mature gp120. J Immunol. 1989 Sep 15;143(6):1832–1836. [PubMed] [Google Scholar]
- Thomas E. K., Weber J. N., McClure J., Clapham P. R., Singhal M. C., Shriver M. K., Weiss R. A. Neutralizing monoclonal antibodies to the AIDS virus. AIDS. 1988 Feb;2(1):25–29. doi: 10.1097/00002030-198802000-00004. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Ross E. K., Buckler-White A. J., Theodore T. S., Martin M. A. Functional interaction of constant and variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3595–3600. doi: 10.1128/jvi.63.9.3595-3600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R., Thali M., Tilley S., Pinter A., Posner M., Ho D., Robinson J., Sodroski J. Relationship of the human immunodeficiency virus type 1 gp120 third variable loop to a component of the CD4 binding site in the fourth conserved region. J Virol. 1992 Dec;66(12):6997–7004. doi: 10.1128/jvi.66.12.6997-7004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]