Abstract
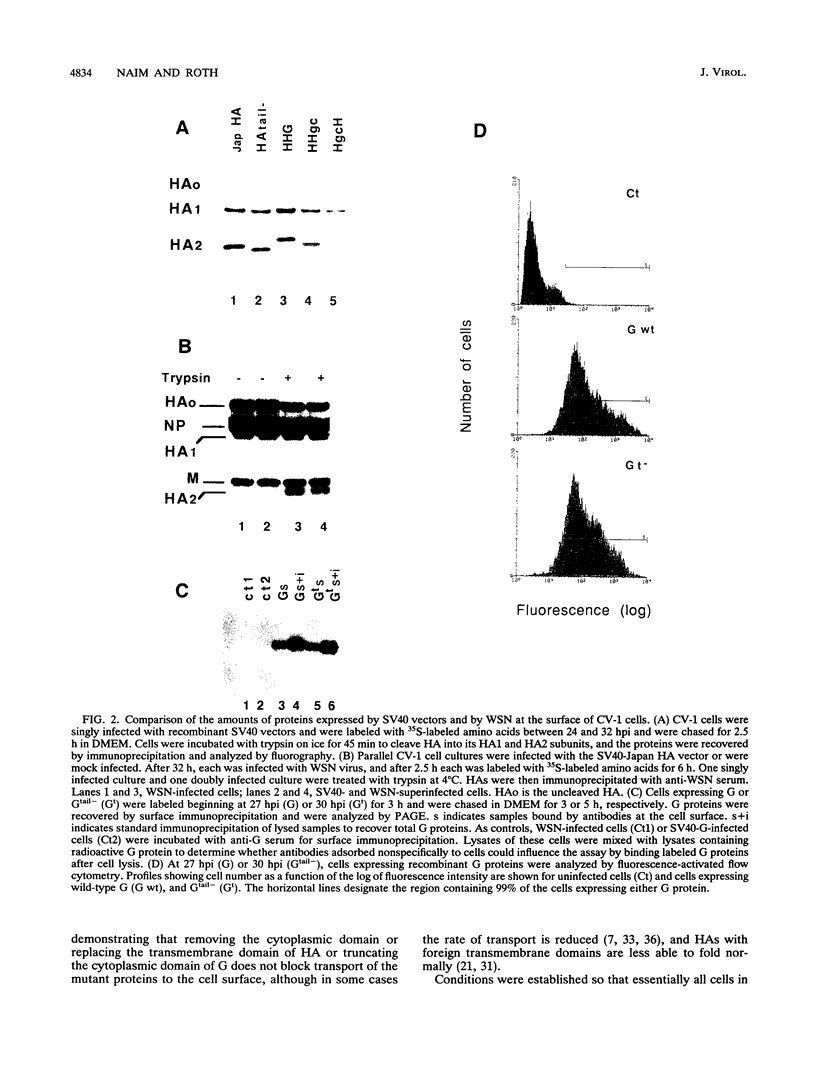
The ability of mutant or chimeric A/Japan hemagglutinins (HAs) to compete for space in the envelope of A/WSN influenza viruses was investigated with monkey kidney fibroblasts that were infected with recombinant simian virus 40 vectors expressing the Japan proteins and superinfected with A/WSN influenza virus. Wild-type Japan HA assembled into virions as well as WSN HA did. Japan HA lacking its cytoplasmic sequences, HAtail-, was incorporated into influenza virions at half the efficiency of wild-type Japan HA. Chimeric HAs containing the 11 cytoplasmic amino acids of the herpes simplex virus type 1gC glycoprotein or the 29 cytoplasmic amino acids of the vesicular stomatitis virus G protein were incorporated into virions at less than 1% the efficiency of HAtail-. Thus, the cytoplasmic domain of HA was not required for the selection process; however, foreign cytoplasmic sequences, even short ones, were excluded. A chimeric HA having the gC transmembrane domain and the HA cytoplasmic domain (HgCH) was incorporated at 4% the efficiency of HAtail-. When expressed from simian virus 40 recombinants in this system, vesicular stomatitis virus G protein with or without (Gtail-) its cytoplasmic domain was essentially excluded from influenza virions. Taken together, these data indicate that the HA transmembrane domain is required for incorporation of HA into influenza virions. The slightly more efficient incorporation of HgCH than G or Gtail- could indicate that the region important for assembling HA into virions extends into part of the cytoplasmic domain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulay F., Doms R. W., Webster R. G., Helenius A. Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol. 1988 Mar;106(3):629–639. doi: 10.1083/jcb.106.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Characterization of the soluble glycoprotein released from vesicular stomatitis virus-infected cells. J Virol. 1983 Jan;45(1):80–90. doi: 10.1128/jvi.45.1.80-90.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Roth M. G., Hunter E. A chimeric avian retrovirus containing the influenza virus hemagglutinin gene has an expanded host range. J Virol. 1992 Dec;66(12):7374–7382. doi: 10.1128/jvi.66.12.7374-7382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Roth M. G., Sambrook J., Gething M. J. Mutations in the cytoplasmic domain of the influenza virus hemagglutinin affect different stages of intracellular transport. J Cell Biol. 1985 Mar;100(3):704–714. doi: 10.1083/jcb.100.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Palese P. High-efficiency formation of influenza virus transfectants. J Virol. 1991 May;65(5):2711–2713. doi: 10.1128/jvi.65.5.2711-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- GOTLIEB T., HIRST G. K. The experimental production of combination forms of virus. III. The formation of doubly antigenic particles from influenza A and B virus and a study of the ability of individual particles of X virus to yield two separate strains. J Exp Med. 1954 Apr 1;99(4):307–320. doi: 10.1084/jem.99.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANOFF A., HIRST G. K. Experimental production of combination forms of virus. IV. Mixed influenza A-Newcastle disease virus infections. Proc Soc Exp Biol Med. 1954 May;86(1):84–88. doi: 10.3181/00379727-86-21016. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Grünberg J., Kruppa A., Paschen P., Kruppa J. Intracellular formation of two soluble glycoproteins in BHK cells infected with vesicular stomatitis virus serotype New Jersey. Virology. 1991 Feb;180(2):678–686. doi: 10.1016/0042-6822(91)90081-l. [DOI] [PubMed] [Google Scholar]
- HIRST G. K., GOTLIEB T. The experimental production of combination forms of virus. I. Occurrence of combination forms after simultaneous inoculation of the allantoic sac with two distinct strains of influenza virus. J Exp Med. 1953 Jul;98(1):41–52. doi: 10.1084/jem.98.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey P. G., Compans R. W., Zebedee S. L., Lamb R. A. Expression of the influenza A virus M2 protein is restricted to apical surfaces of polarized epithelial cells. J Virol. 1992 Sep;66(9):5542–5552. doi: 10.1128/jvi.66.9.5542-5552.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving R. A., Ghosh H. P. Shedding of vesicular stomatitis virus soluble glycoprotein by removal of carboxy-terminal peptide. J Virol. 1982 Apr;42(1):322–325. doi: 10.1128/jvi.42.1.322-325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J., Elson E. L. Fluorescence photobleaching recovery measurements reveal differences in envelopment of Sindbis and vesicular stomatitis viruses. Cell. 1981 Feb;23(2):423–431. doi: 10.1016/0092-8674(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits J., Shia S. P., Ktistakis N., Lee M. S., Bird C., Roth M. G. The effects of foreign transmembrane domains on the biosynthesis of the influenza virus hemagglutinin. J Biol Chem. 1990 Mar 15;265(8):4760–4767. [PubMed] [Google Scholar]
- Little S. P., Huang A. S. Shedding of the glycoprotein from vesicular stomatitis virus-infected cells. J Virol. 1978 Aug;27(2):330–339. doi: 10.1128/jvi.27.2.330-339.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Weiss R. A. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979 Apr;30(1):177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Simons K. The budding mechanism of spikeless vesicular stomatitis virus particles. EMBO J. 1986 Aug;5(8):1913–1920. doi: 10.1002/j.1460-2075.1986.tb04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim H. Y., Amarneh B., Ktistakis N. T., Roth M. G. Effects of altering palmitylation sites on biosynthesis and function of the influenza virus hemagglutinin. J Virol. 1992 Dec;66(12):7585–7588. doi: 10.1128/jvi.66.12.7585-7588.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobusawa E., Aoyama T., Kato H., Suzuki Y., Tateno Y., Nakajima K. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology. 1991 Jun;182(2):475–485. doi: 10.1016/0042-6822(91)90588-3. [DOI] [PubMed] [Google Scholar]
- Pattnaik A. K., Brown D. J., Nayak D. P. Formation of influenza virus particles lacking hemagglutinin on the viral envelope. J Virol. 1986 Dec;60(3):994–1001. doi: 10.1128/jvi.60.3.994-1001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W., Giusti L., Davis A. R., Nayak D. P., Gething M. J., Sambrook J. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell. 1983 Jun;33(2):435–443. doi: 10.1016/0092-8674(83)90425-7. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Doyle C., Sambrook J., Gething M. J. Heterologous transmembrane and cytoplasmic domains direct functional chimeric influenza virus hemagglutinins into the endocytic pathway. J Cell Biol. 1986 Apr;102(4):1271–1283. doi: 10.1083/jcb.102.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Simpson D. A., Lamb R. A. Alterations to influenza virus hemagglutinin cytoplasmic tail modulate virus infectivity. J Virol. 1992 Feb;66(2):790–803. doi: 10.1128/jvi.66.2.790-803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Brewer C. B., Roth M. G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993 Feb 15;268(5):3313–3320. [PubMed] [Google Scholar]
- Weiss R. A., Boettiger D., Murphy H. M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977 Feb;76(2):808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Whitt M. A., Chong L., Rose J. K. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J Virol. 1989 Sep;63(9):3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C., Reitz M. S., Okayama H., Eiden M. V. Formation of infectious hybrid virions with gibbon ape leukemia virus and human T-cell leukemia virus retroviral envelope glycoproteins and the gag and pol proteins of Moloney murine leukemia virus. J Virol. 1989 May;63(5):2374–2378. doi: 10.1128/jvi.63.5.2374-2378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Mechanism of formation of pseudotypes between vesicular stomatitis virus and murine leukemia virus. Cell. 1977 Jul;11(3):505–511. doi: 10.1016/0092-8674(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Young J. A., Bates P., Willert K., Varmus H. E. Efficient incorporation of human CD4 protein into avian leukosis virus particles. Science. 1990 Dec 7;250(4986):1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]
- Zhao H., Garoff H. Role of cell surface spikes in alphavirus budding. J Virol. 1992 Dec;66(12):7089–7095. doi: 10.1128/jvi.66.12.7089-7095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závada J., Rosenbergová M. Phenotypic mixing of vesicular stomatitis virus with fowl plague virus. Acta Virol. 1972 Mar;16(2):103–114. [PubMed] [Google Scholar]
- de Giuli C., Kawai S., Dales S., Hanafusa H. Absence of surface projections of some noninfectious forms of RSV. Virology. 1975 Jul;66(1):253–260. doi: 10.1016/0042-6822(75)90195-6. [DOI] [PubMed] [Google Scholar]