Abstract
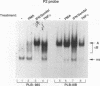
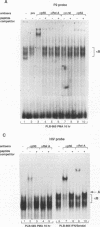
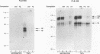
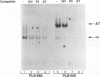
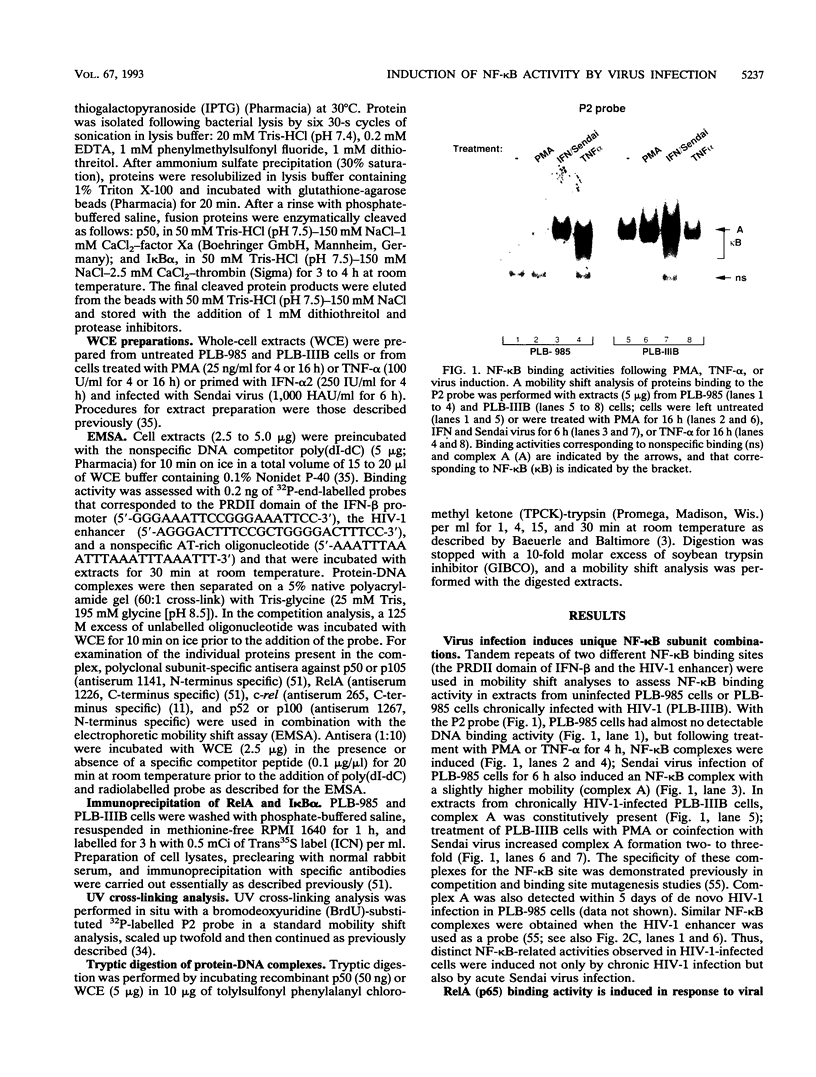
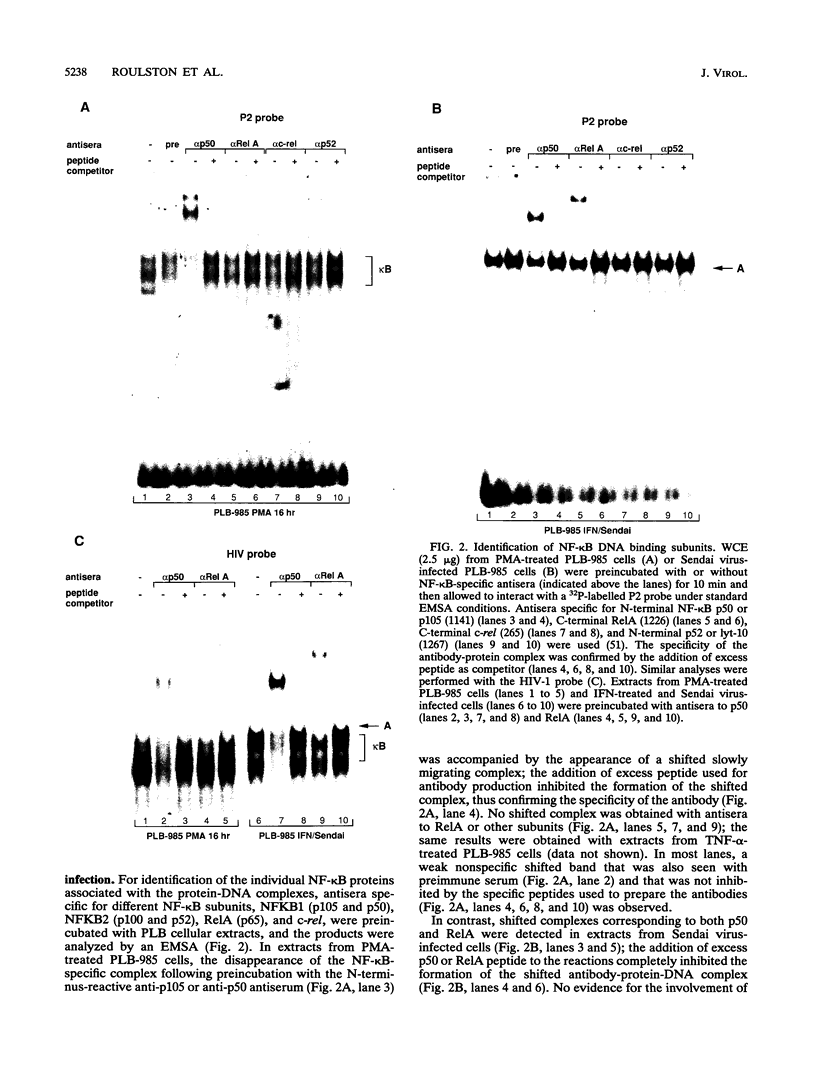
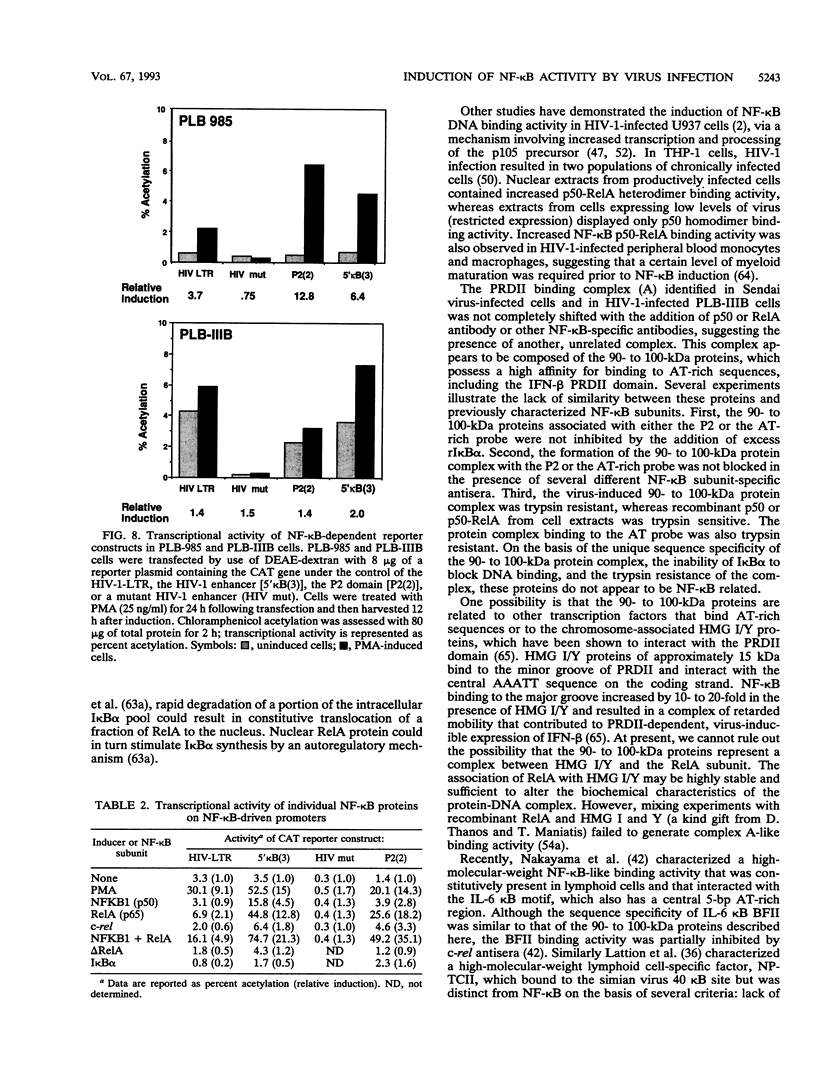
The relationship between human immunodeficiency virus type 1 (HIV-1) infection and the induction of NF-kappa B binding activity was examined in a myeloid cell model of HIV-1 infection derived from the PLB-985 cell line. Chronic infection of PLB-985 cells led to increased monocyte-specific surface marker expression, increased c-fms gene transcription, and morphological alterations consistent with differentiation along the monocytic pathway. PLB-IIIB cells displayed a constitutive NF-kappa B-like binding activity that was distinct from that induced by tumor necrosis factor alpha or phorbol 12-myristate 13-acetate treatment of the parental PLB-985 cell line. This unique DNA binding activity consisted of proteins of 70, 90, and 100 kDa with a high degree of binding specificity for the NF-kappa B site within the PRDII domain of beta interferon. In this report, we characterize the nature of these proteins and demonstrate that binding of these proteins is also induced following Sendai paramyxovirus infection. The 70-kDa protein corresponds to the NF-kappa B RelA (p65) subunit, which is activated in response to an acute paramyxovirus infection or a chronic HIV-1 infection. Virus infection does not appear to alter the amount of RelA (p65) or NFKB1 (p50) but rather affects the capacity of I kappa B alpha to sequester RelA (p65), therefore leading to constitutive levels of RelA DNA binding activity and to increased levels of NF-kappa B-dependent gene activity. The virally induced 90- to 100-kDa proteins have a distinct binding specificity for the PRDII domain and an AT-rich sequence but do not cross-react with NF-kappa B subunit-specific antisera directed against NFKB1 (p105 or p50), NFKB2 (p100 or p52), RelA (p65), or c-rel. DNA binding of the 90- to 100-kDa proteins was not inhibited by recombinant I kappa B alpha/MAD-3 and was resistant to tryptic digestion, suggesting that these proteins may not be NF-kappa B related. Transient cotransfection experiments demonstrated that RelA and NFKB1 expression maximally stimulated HIV-1 LTR- and NF-kappa B-dependent reporter genes; differences in NF-kappa B-like binding activity were also reflected in higher constitutive levels of NF-kappa B-regulated gene expression in HIV-1-infected myeloid cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Brugger W., Kunze R., Stille W., von Briesen H. Defective monocyte to macrophage maturation in human immunodeficiency virus infection. Res Virol. 1990 Mar-Apr;141(2):217–224. doi: 10.1016/0923-2516(90)90024-d. [DOI] [PubMed] [Google Scholar]
- Bachelerie F., Alcami J., Arenzana-Seisdedos F., Virelizier J. L. HIV enhancer activity perpetuated by NF-kappa B induction on infection of monocytes. Nature. 1991 Apr 25;350(6320):709–712. doi: 10.1038/350709a0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Dixon E. P., Peffer N. J., Bogerd H., Doerre S., Stein B., Greene W. C. The 65-kDa subunit of human NF-kappa B functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1875–1879. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- Blank V., Kourilsky P., Israël A. NF-kappa B and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem Sci. 1992 Apr;17(4):135–140. doi: 10.1016/0968-0004(92)90321-y. [DOI] [PubMed] [Google Scholar]
- Bose H. R., Jr The Rel family: models for transcriptional regulation and oncogenic transformation. Biochim Biophys Acta. 1992 Sep 14;1114(1):1–17. doi: 10.1016/0304-419x(92)90002-g. [DOI] [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Brownell E., Mittereder N., Rice N. R. A human rel proto-oncogene cDNA containing an Alu fragment as a potential coding exon. Oncogene. 1989 Jul;4(7):935–942. [PubMed] [Google Scholar]
- D'Addario M., Roulston A., Wainberg M. A., Hiscott J. Coordinate enhancement of cytokine gene expression in human immunodeficiency virus type 1-infected promonocytic cells. J Virol. 1990 Dec;64(12):6080–6089. doi: 10.1128/jvi.64.12.6080-6089.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario M., Wainberg M. A., Hiscott J. Activation of cytokine genes in HIV-1 infected myelomonoblastic cells by phorbol ester and tumor necrosis factor. J Immunol. 1992 Feb 15;148(4):1222–1229. [PubMed] [Google Scholar]
- Folks T. M., Kessler S. W., Orenstein J. M., Justement J. S., Jaffe E. S., Fauci A. S. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988 Nov 11;242(4880):919–922. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- Franzoso G., Bours V., Park S., Tomita-Yamaguchi M., Kelly K., Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-kappa B-mediated inhibition. Nature. 1992 Sep 24;359(6393):339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Nolan G. P., Ghosh S., Baltimore D. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-kappa B. Genes Dev. 1992 May;6(5):775–787. doi: 10.1101/gad.6.5.775. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Macé K. Regulation of HIV1 replication in promonocytic U937 cells. Res Virol. 1990 Mar-Apr;141(2):259–265. doi: 10.1016/0923-2516(90)90030-m. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Gill P. S. Pathogenesis of HIV-related malignancies. Curr Opin Oncol. 1991 Oct;3(5):867–871. doi: 10.1097/00001622-199110000-00010. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D. NF-kappa B, KBF1, dorsal, and related matters. Cell. 1990 Sep 7;62(5):841–843. doi: 10.1016/0092-8674(90)90257-f. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Alper D., Cohen L., Leblanc J. F., Sportza L., Wong A., Xanthoudakis S. Induction of human interferon gene expression is associated with a nuclear factor that interacts with the NF-kappa B site of the human immunodeficiency virus enhancer. J Virol. 1989 Jun;63(6):2557–2566. doi: 10.1128/jvi.63.6.2557-2566.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Kakizuka A., Verma I. M. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell. 1992 Mar 20;68(6):1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Ransone L. J., Bengal E., Hunter T., Verma I. M. c-rel activates but v-rel suppresses transcription from kappa B sites. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3715–3719. doi: 10.1073/pnas.88.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Rashid D., Davis N., Bose H. R., Jr, Verma I. M. Direct association of pp40/I kappa B beta with rel/NF-kappa B transcription factors: role of ankyrin repeats in the inhibition of DNA binding activity. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4333–4337. doi: 10.1073/pnas.89.10.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Duckett C. S., Wamsley P., Zhang Q., Chiao P., Nabel G., McKeithan T. W., Baeuerle P. A., Verma I. M. The proto-oncogene bcl-3 encodes an I kappa B protein. Genes Dev. 1992 Dec;6(12A):2352–2363. doi: 10.1101/gad.6.12a.2352. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Inoue J., Davis N., Link E., Baeuerle P. A., Bose H. R., Jr, Verma I. M. The rel-associated pp40 protein prevents DNA binding of Rel and NF-kappa B: relationship with I kappa B beta and regulation by phosphorylation. Genes Dev. 1991 Aug;5(8):1464–1476. doi: 10.1101/gad.5.8.1464. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Ruben S. M., Rosen C. A. Selection of optimal kappa B/Rel DNA-binding motifs: interaction of both subunits of NF-kappa B with DNA is required for transcriptional activation. Mol Cell Biol. 1992 Oct;12(10):4412–4421. doi: 10.1128/mcb.12.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste J., Cohen L., Hiscott J. NF-kappa B activity in T cells stably expressing the Tax protein of human T cell lymphotropic virus type I. Virology. 1991 Oct;184(2):553–562. doi: 10.1016/0042-6822(91)90425-b. [DOI] [PubMed] [Google Scholar]
- Lacoste J., D'Addario M., Roulston A., Wainberg M. A., Hiscott J. Cell-specific differences in activation of NF-kappa B regulatory elements of human immunodeficiency virus and beta interferon promoters by tumor necrosis factor. J Virol. 1990 Oct;64(10):4726–4734. doi: 10.1128/jvi.64.10.4726-4734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattion A. L., Espel E., Reichenbach P., Fromental C., Bucher P., Israël A., Baeuerle P., Rice N. R., Nabholz M. Characterization of a new tissue-specific transcription factor binding to the simian virus 40 enhancer TC-II (NF-kappa B) element. Mol Cell Biol. 1992 Nov;12(11):5217–5227. doi: 10.1128/mcb.12.11.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc J. F., Cohen L., Rodrigues M., Hiscott J. Synergism between distinct enhanson domains in viral induction of the human beta interferon gene. Mol Cell Biol. 1990 Aug;10(8):3987–3993. doi: 10.1128/mcb.10.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locardi C., Petrini C., Boccoli G., Testa U., Dieffenbach C., Buttò S., Belardelli F. Increased human immunodeficiency virus (HIV) expression in chronically infected U937 cells upon in vitro differentiation by hydroxyvitamin D3: roles of interferon and tumor necrosis factor in regulation of HIV production. J Virol. 1990 Dec;64(12):5874–5882. doi: 10.1128/jvi.64.12.5874-5882.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Koyanagi Y., Chen I. S. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989 Oct;63(10):4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R., Hatada E. N., Hohmann H. P., Haiker M., Bartsch C., Röthlisberger U., Lahm H. W., Schlaeger E. J., van Loon A. P., Scheidereit C. Cloning of the DNA-binding subunit of human nuclear factor kappa B: the level of its mRNA is strongly regulated by phorbol ester or tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):966–970. doi: 10.1073/pnas.88.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. A lymphoid cell-specific nuclear factor containing c-Rel-like proteins preferentially interacts with interleukin-6 kappa B-related motifs whose activities are repressed in lymphoid cells. Mol Cell Biol. 1992 Apr;12(4):1736–1746. doi: 10.1128/mcb.12.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R., Klement J. F., Ruben S. M., Higgins K. A., Rosen C. A. Identification of a naturally occurring transforming variant of the p65 subunit of NF-kappa B. Science. 1992 Apr 17;256(5055):367–370. doi: 10.1126/science.256.5055.367. [DOI] [PubMed] [Google Scholar]
- Neri A., Chang C. C., Lombardi L., Salina M., Corradini P., Maiolo A. T., Chaganti R. S., Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991 Dec 20;67(6):1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Pautrat G., Suzan M., Salaun D., Corbeau P., Allasia C., Morel G., Filippi P. Human immunodeficiency virus type 1 infection of U937 cells promotes cell differentiation and a new pathway of viral assembly. Virology. 1990 Dec;179(2):749–758. doi: 10.1016/0042-6822(90)90142-e. [DOI] [PubMed] [Google Scholar]
- Paya C. V., Ten R. M., Bessia C., Alcami J., Hay R. T., Virelizier J. L. NF-kappa B-dependent induction of the NF-kappa B p50 subunit gene promoter underlies self-perpetuation of human immunodeficiency virus transcription in monocytic cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7826–7830. doi: 10.1073/pnas.89.16.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N. D., Schmid R. M., Duckett C. S., Leung K., Rice N. R., Nabel G. J. Distinct combinations of NF-kappa B subunits determine the specificity of transcriptional activation. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1529–1533. doi: 10.1073/pnas.89.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- Raziuddin, Mikovits J. A., Calvert I., Ghosh S., Kung H. F., Ruscetti F. W. Negative regulation of human immunodeficiency virus type 1 expression in monocytes: role of the 65-kDa plus 50-kDa NF-kappa B dimer. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9426–9430. doi: 10.1073/pnas.88.21.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., MacKichan M. L., Israël A. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell. 1992 Oct 16;71(2):243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- Rivière Y., Blank V., Kourilsky P., Israël A. Processing of the precursor of NF-kappa B by the HIV-1 protease during acute infection. Nature. 1991 Apr 18;350(6319):625–626. doi: 10.1038/350625a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenesis of HIV infection. FASEB J. 1991 Jul;5(10):2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- Roulston A., D'Addario M., Boulerice F., Caplan S., Wainberg M. A., Hiscott J. Induction of monocytic differentiation and NF-kappa B-like activities by human immunodeficiency virus 1 infection of myelomonoblastic cells. J Exp Med. 1992 Mar 1;175(3):751–763. doi: 10.1084/jem.175.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S. M., Dillon P. J., Schreck R., Henkel T., Chen C. H., Maher M., Baeuerle P. A., Rosen C. A. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science. 1991 Mar 22;251(5000):1490–1493. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Klement J. F., Coleman T. A., Maher M., Chen C. H., Rosen C. A. I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev. 1992 May;6(5):745–760. doi: 10.1101/gad.6.5.745. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Narayanan R., Klement J. F., Chen C. H., Rosen C. A. Functional characterization of the NF-kappa B p65 transcriptional activator and an alternatively spliced derivative. Mol Cell Biol. 1992 Feb;12(2):444–454. doi: 10.1128/mcb.12.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bull P., Takamiya M., Bours V., Siebenlist U., Dobrzanski P., Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992 Feb;12(2):674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Suzan M., Salaun D., Neuveut C., Spire B., Hirsch I., Le Bouteiller P., Querat G., Sire J. Induction of NF-KB during monocyte differentiation by HIV type 1 infection. J Immunol. 1991 Jan 1;146(1):377–383. [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Tucker K. A., Lilly M. B., Heck L., Jr, Rado T. A. Characterization of a new human diploid myeloid leukemia cell line (PLB-985) with granulocytic and monocytic differentiating capacity. Blood. 1987 Aug;70(2):372–378. [PubMed] [Google Scholar]
- Turpin J. A., Vargo M., Meltzer M. S. Enhanced HIV-1 replication in retinoid-treated monocytes. Retinoid effects mediated through mechanisms related to cell differentiation and to a direct transcriptional action on viral gene expression. J Immunol. 1992 Apr 15;148(8):2539–2546. [PubMed] [Google Scholar]
- Urban M. B., Baeuerle P. A. The 65-kD subunit of NF-kappa B is a receptor for I kappa B and a modulator of DNA-binding specificity. Genes Dev. 1990 Nov;4(11):1975–1984. doi: 10.1101/gad.4.11.1975. [DOI] [PubMed] [Google Scholar]
- Urban M. B., Baeuerle P. A. The role of the p50 and p65 subunits of NF-kappa B in the recognition of cognate sequences. New Biol. 1991 Mar;3(3):279–288. [PubMed] [Google Scholar]
- Urban M. B., Schreck R., Baeuerle P. A. NF-kappa B contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 1991 Jul;10(7):1817–1825. doi: 10.1002/j.1460-2075.1991.tb07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulczyn F. G., Naumann M., Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-kappa B. Nature. 1992 Aug 13;358(6387):597–599. doi: 10.1038/358597a0. [DOI] [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]