Abstract
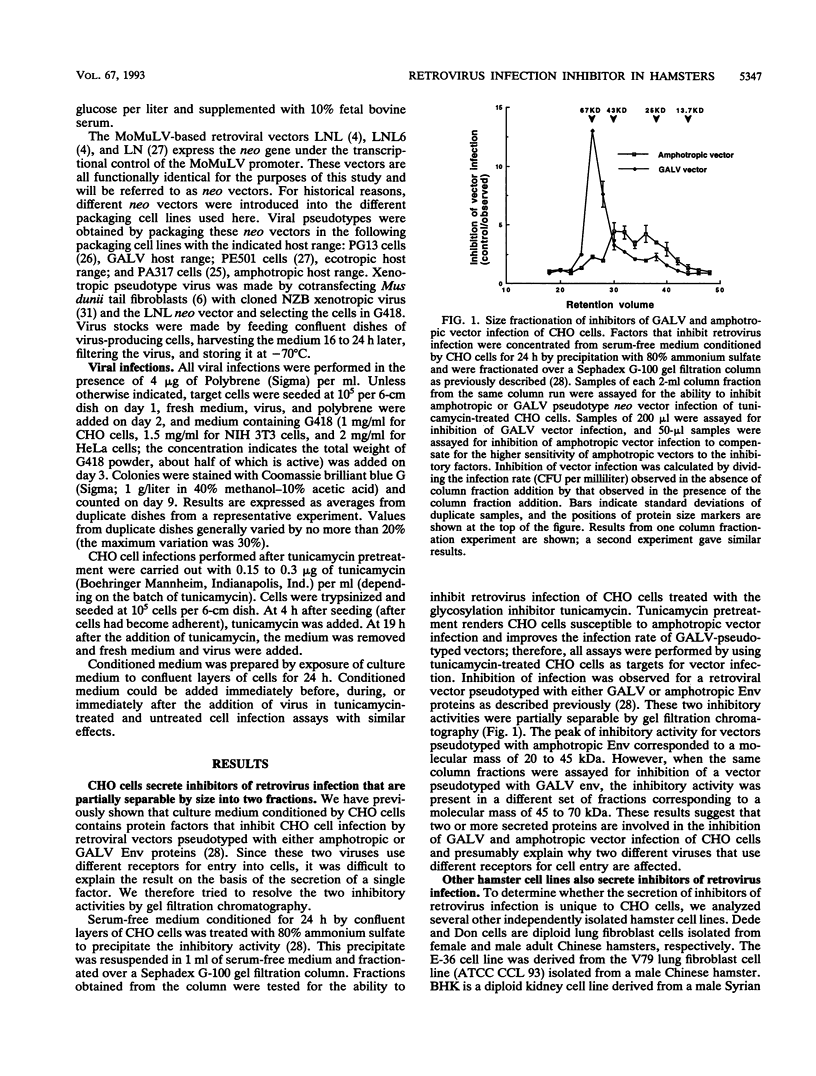
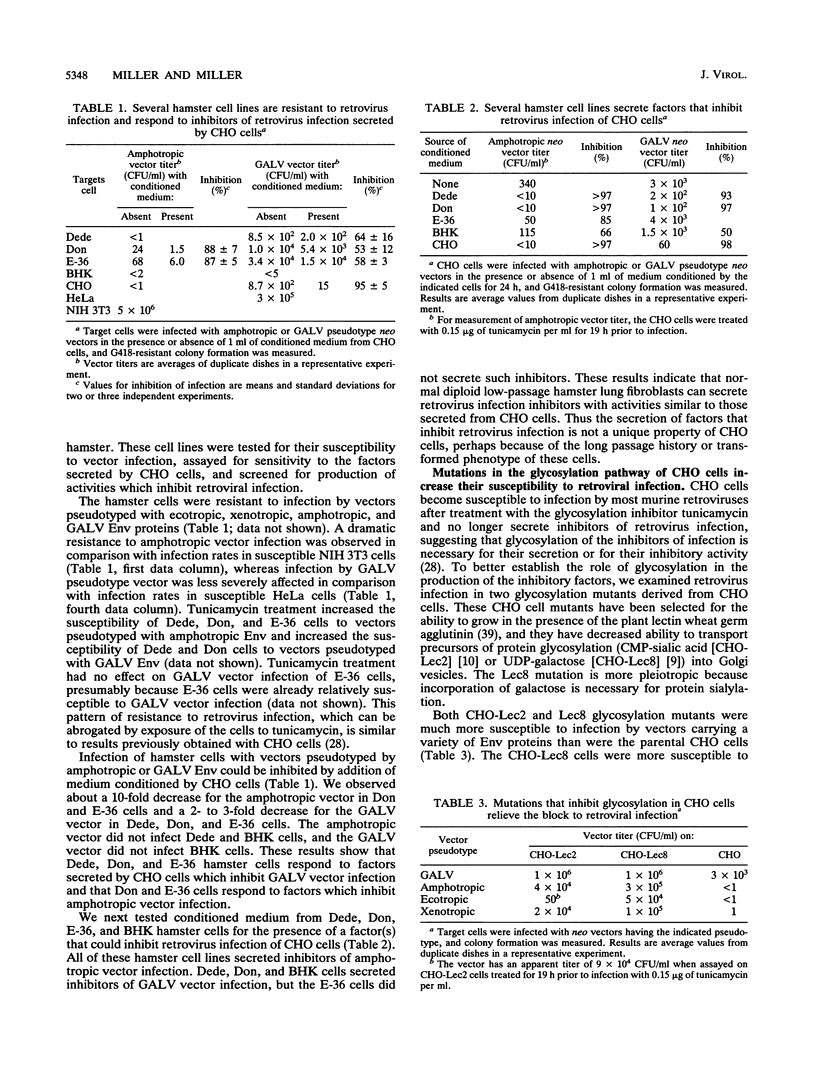
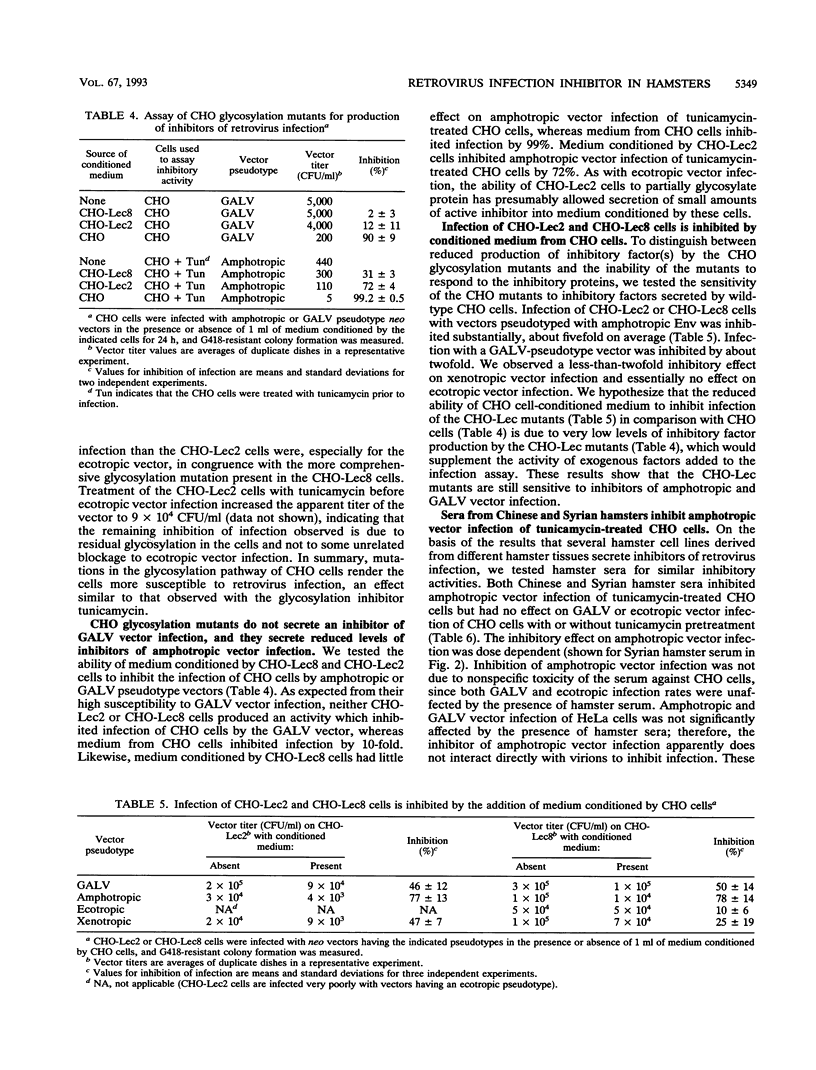
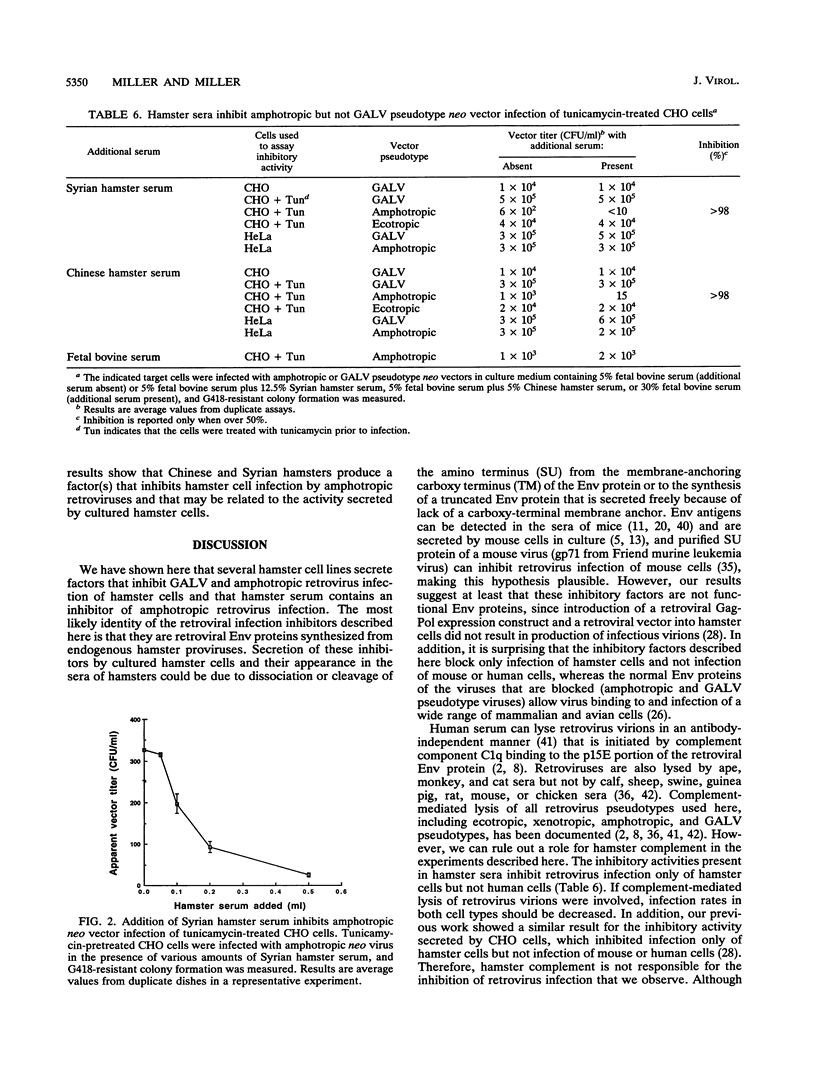
We have previously shown that Chinese hamster ovary (CHO) cells are resistant to infection by gibbon ape leukemia virus and amphotropic pseudotype retroviral vectors because of the secretion of factors that inhibit retrovirus infection. Such factors were not secreted by any mouse or human cell lines tested. Secretion of the inhibitors and resistance to infection are abrogated by treatment of CHO cells with the glycosylation inhibitor tunicamycin. Here we show that the inhibitory activities against gibbon ape leukemia virus and amphotropic viruses are partially separable and that glycosylation mutations in CHO cells mimic the effects of tunicamycin treatment. We find that several hamster cell lines derived from both Chinese and Syrian hamsters secrete inhibitors of retrovirus infection, showing that these inhibitors are not unique to the CHO cell line. Inhibitory factors are also present in the sera of Chinese and Syrian hamsters but were not detected in bovine serum. These results suggest the presence of specific factors that function to inhibit retrovirus infection in hamsters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B., Robison H., Varmus H. E., Bishop J. M. Analysis of endogenous avian retrovirus DNA and RNA: viral and cellular determinants of retrovirus gene expression. Virology. 1981 Oct 15;114(1):8–22. doi: 10.1016/0042-6822(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Bartholomew R. M., Esser A. F., Müller-Eberhard H. J. Lysis of oncornaviruses by human serum. Isolation of the viral complement (C1) receptor and identification as p15E. J Exp Med. 1978 Mar 1;147(3):844–853. doi: 10.1084/jem.147.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Ruscetti S., Ali I., Haapala D. K., Rein A. Normal DBA/2 mouse cells synthesize a glycoprotein which interferes with MCF virus infection. Virology. 1982 Nov;123(1):139–151. doi: 10.1016/0042-6822(82)90301-4. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P., Langlois A. J., Schäfer W. Polypeptides of mammalian oncornaviruses. IV. Structural components of murine leukemia virus released as soluble antigens in cell culture. Virology. 1975 Dec;68(2):550–555. doi: 10.1016/0042-6822(75)90297-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Jensen F. C., Welsh R. M., Jr, Oldstone M. B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976 Oct 1;144(4):970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Deutscher S. L., Nuwayhid N., Stanley P., Briles E. I., Hirschberg C. B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984 Dec;39(2 Pt 1):295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Ihle J. N., Bolognesi D. P., Schäfer W. Inactivation of murine xenotropic oncornavirus by normal mouse sera is not immunoglobulin-mediated. Virology. 1976 May;71(1):346–351. doi: 10.1016/0042-6822(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Thiel H. J., Ihle J. N., Lee J. C., Elder J. H. Detection of a recombinant murine leukemia virus-related glycoprotein on virus-negative thymoma cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1920–1924. doi: 10.1073/pnas.78.3.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Kozak C. A., O'Brien S. J. The Lake Casitas wild mouse: evolving genetic resistance to retroviral disease. Trends Genet. 1991 Jan;7(1):22–27. doi: 10.1016/0168-9525(91)90017-k. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Yetter R. A., Morse H. C., 3rd A mouse gene on chromosome 5 that restricts infectivity of mink cell focus-forming recombinant murine leukemia viruses. J Exp Med. 1983 Jul 1;158(1):16–24. doi: 10.1084/jem.158.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Odaka T. Expression of Fv-4r allele in hematopoietic cells from G mice resistant to Friend leukemia virus. Int J Cancer. 1979 Apr 15;23(4):514–518. doi: 10.1002/ijc.2910230412. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Chermann J. C., Jasmin C., Raynaud M. Mise en évidence d'une substance à effet antiviral dans le milieu de culture de cellules de souris NZ. C R Acad Sci Hebd Seances Acad Sci D. 1973 Oct 8;277(14):1421–1423. [PubMed] [Google Scholar]
- Levy J. A., Ihle J. N., Oleszko O., Barnes R. D. Virus-specific neutralization by a soluble non-immunoglobulin factor found naturally in normal mouse sera. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5071–5075. doi: 10.1073/pnas.72.12.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M., Yoshikura H. Construction and characterization of the recombinant Moloney murine leukemia viruses bearing the mouse Fv-4 env gene. J Virol. 1990 Mar;64(3):1033–1043. doi: 10.1128/jvi.64.3.1033-1043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Garcia J. V., von Suhr N., Lynch C. M., Wilson C., Eiden M. V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991 May;65(5):2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller D. G., Miller A. D. Tunicamycin treatment of CHO cells abrogates multiple blocks to retrovirus infection, one of which is due to a secreted inhibitor. J Virol. 1992 Jan;66(1):78–84. doi: 10.1128/jvi.66.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Fischinger P. J., Larrick S. B., Dunlop N. M., Ihle J. N., Frank H., Schäfer W., Bolognesi D. P. Further characterization of the oncornavirus inactivating factor in normal mouse serum. Virology. 1979 Oct 15;98(1):20–34. doi: 10.1016/0042-6822(79)90521-x. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Dunlop N. M., Robey W. G., Callahan R., Fischinger P. J. Lipoprotein-associated oncornavirus-inactivating factor in the genus Mus: effects on murine leukemia viruses of laboratory and exotic mice. Cancer Res. 1987 Feb 1;47(3):667–672. [PubMed] [Google Scholar]
- O'Neill R. R., Buckler C. E., Theodore T. S., Martin M. A., Repaske R. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J Virol. 1985 Jan;53(1):100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. M., Bader J. P., Bassin R. H. Inhibitors of glycosylation reverse retroviral interference. Virology. 1982 May;119(1):185–192. doi: 10.1016/0042-6822(82)90075-7. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Senior A. M., Salazar F. H. Host Susceptibility to endogenous viruses: defective, glycoprotein-expressing proviruses interfere with infections. J Virol. 1981 Dec;40(3):745–751. doi: 10.1128/jvi.40.3.745-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W., Fischinger P. J., Collins J. J., Bolognesi D. P. Role of carbohydrate in biological functions of Friend murine leukemia virus gp71. J Virol. 1977 Jan;21(1):35–40. doi: 10.1128/jvi.21.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin S. A., Benveniste R. E., Todaro G. J. Complement-mediated lysis of type-C virus: effect of primate and human sera on various retroviruses. Int J Cancer. 1978 Jan 15;21(1):6–11. doi: 10.1002/ijc.2910210103. [DOI] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Stanley P. Selection of specific wheat germ agglutinin-resistant (WgaR) phenotypes from Chinese hamster ovary cell populations containing numerous lecR genotypes. Mol Cell Biol. 1981 Aug;1(8):687–696. doi: 10.1128/mcb.1.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977 Jul;3(4):391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Oncornavirus envelope glycoprotein in serum of mice. Virology. 1976 Nov;75(1):130–144. doi: 10.1016/0042-6822(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Cooper N. R., Jensen F. C., Oldstone M. B. Human serum lyses RNA tumour viruses. Nature. 1975 Oct 16;257(5527):612–614. doi: 10.1038/257612a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Jensen F. C., Cooper N. R., Oldstone M. B. Inactivation of lysis of oncornaviruses by human serum. Virology. 1976 Oct 15;74(2):432–440. doi: 10.1016/0042-6822(76)90349-4. [DOI] [PubMed] [Google Scholar]



