Abstract
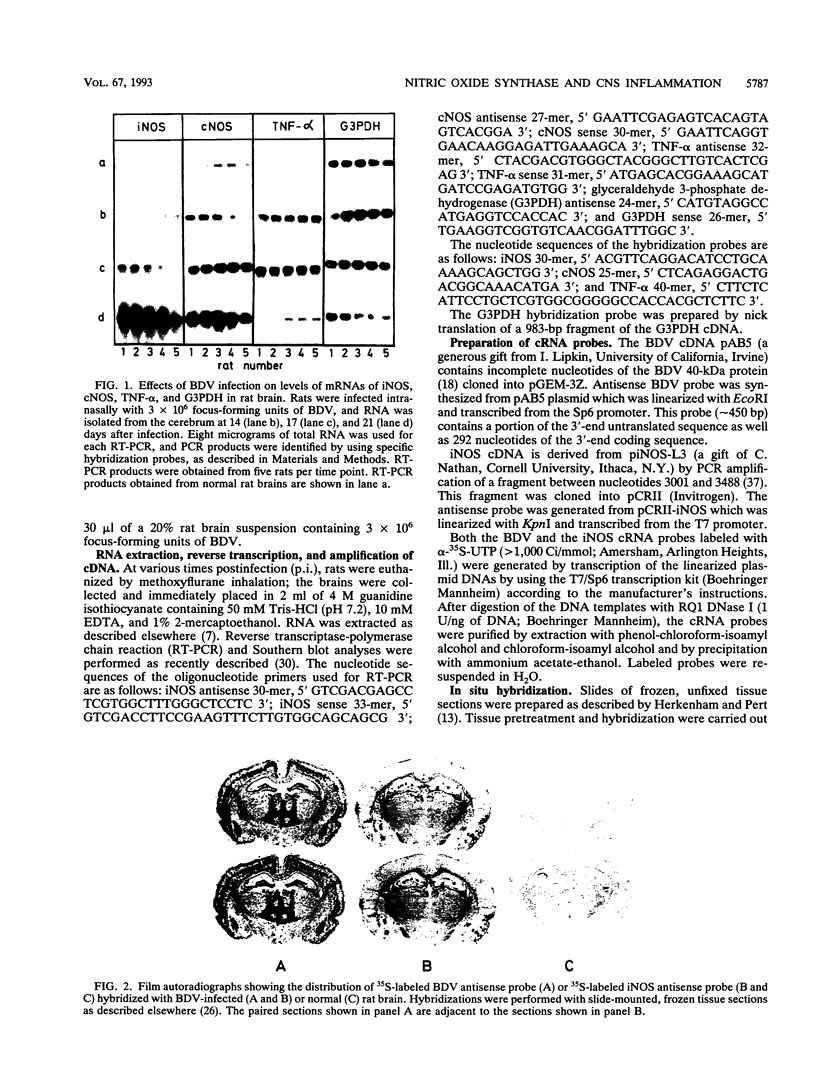
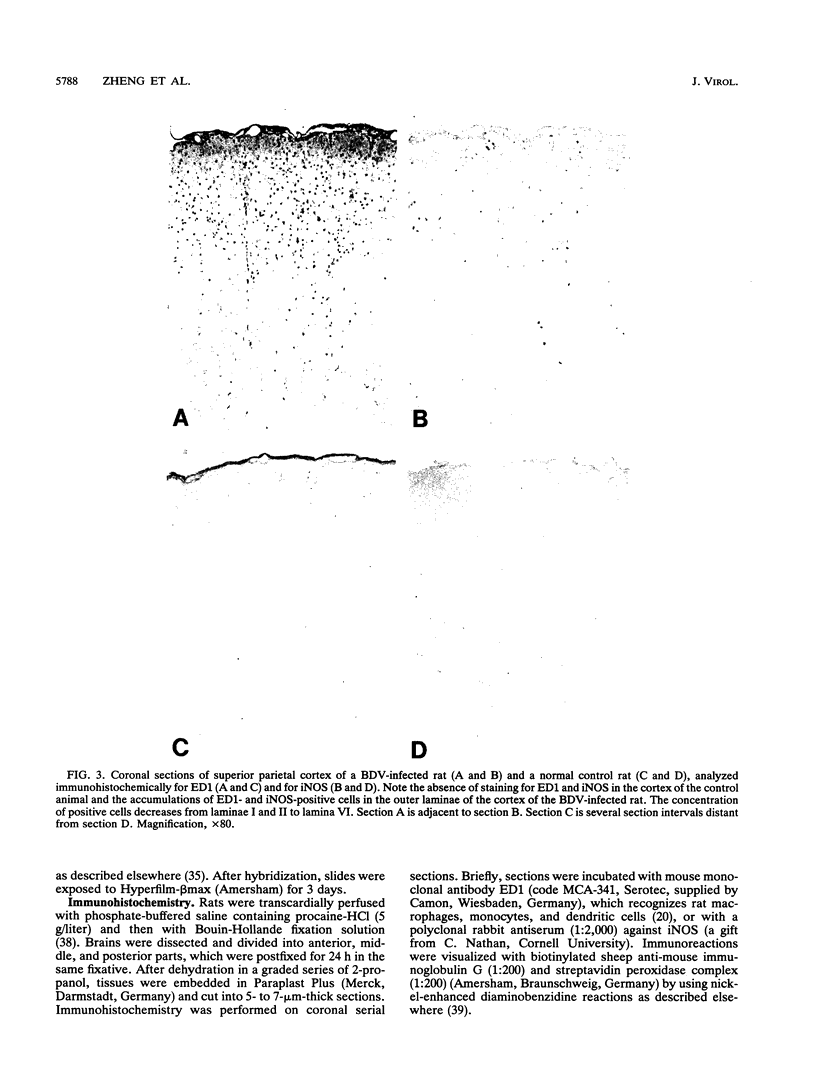
The putative role of nitric oxide in the neuropathogenesis of Borna disease was investigated by determining changes in the expression of inducible nitric oxide synthase (iNOS) mRNA and constitutively expressed NOS (cNOS) mRNA in brains of Borna disease virus (BDV)-infected rats. iNOS mRNA was not detected in normal rat brain but was identified in BDV-infected brain at 14 days postinfection (p.i.), reaching maximum levels at 21 days p.i., when neurological signs and inflammatory reactions in the brain were also at a peak. cNOS mRNA was expressed in both normal brain and infected brain, increasing markedly at 17 days p.i. and reaching a peak at 21 days p.i. In situ hybridization analysis revealed iNOS mRNA in some, but not all, BDV-infected regions of the brain, particularly in the basolateral cortex and the hippocampus. iNOS-positive cells, as identified immunohistologically, were preferentially localized in perivascular areas of the hippocampus and in outer cortical layers. These iNOS-positive cells resembled monocytes/macrophages in morphology and distribution pattern but were significantly fewer. The correlation of iNOS and cNOS mRNA expression with the development of neurological disease, as well as the enhanced expression of iNOS within brain regions with inflammatory lesions, strongly suggests that NO may contribute to pathogenesis of Borna disease.
Full text
PDF

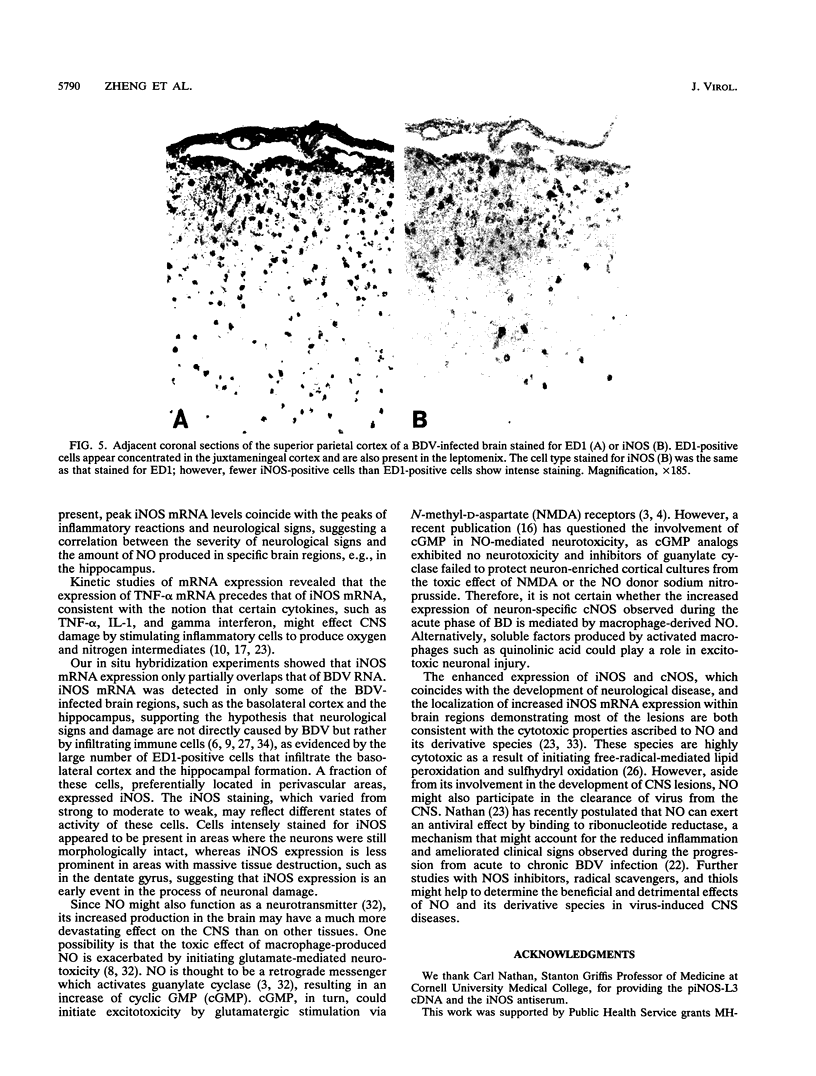

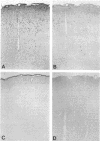
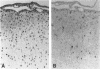
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P. B., Perry V. H., Gordon S. The acute inflammatory response to lipopolysaccharide in CNS parenchyma differs from that in other body tissues. Neuroscience. 1992;48(1):169–186. doi: 10.1016/0306-4522(92)90347-5. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Duchala C. S., Narayan O. Borna disease. An immunopathologic response to viral infection in the CNS. Ann N Y Acad Sci. 1988;540:661–662. doi: 10.1111/j.1749-6632.1988.tb27204.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschl U., Stitz L., Herzog S., Frese K., Rott R. Determination of immune cells and expression of major histocompatibility complex class II antigen in encephalitic lesions of experimental Borna disease. Acta Neuropathol. 1990;81(1):41–50. doi: 10.1007/BF00662636. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Doherty P. C., Dunlop M. B., Parish C. R., Zinkernagel R. M. Inflammatory process in murine lymphocytic choriomeningitis is maximal in H-2K or H-2D compatible interactions. J Immunol. 1976 Jul;117(1):187–190. [PubMed] [Google Scholar]
- Grant I., Atkinson J. H. Neurogenic and psychogenic behavioral correlates of HIV infection. Res Publ Assoc Res Nerv Ment Dis. 1990;68:291–304. [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982 Aug;2(8):1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevel J. M., White K. A., Marletta M. A. Purification of the inducible murine macrophage nitric oxide synthase. Identification as a flavoprotein. J Biol Chem. 1991 Dec 5;266(34):22789–22791. [PubMed] [Google Scholar]
- Lipkin W. I., Travis G. H., Carbone K. M., Wilson M. C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig H. S., von Brauchitsch K. L., Chan J., Greenberg D. A. Cyclic GMP modulators and excitotoxic injury in cerebral cortical cultures. Brain Res. 1992 Apr 17;577(2):343–346. doi: 10.1016/0006-8993(92)90295-k. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Thibault K. J., Hatalski C. G., Lipkin W. I. Sequence similarity between Borna disease virus p40 and a duplicated domain within the paramyxovirus and rhabdovirus polymerase proteins. J Virol. 1992 Nov;66(11):6572–6577. doi: 10.1128/jvi.66.11.6572-6577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S., Weihe E. Interrelation of peptidergic innervation with mast cells and ED1-positive cells in rat thymus. Brain Behav Immun. 1991 Mar;5(1):55–72. doi: 10.1016/0889-1591(91)90007-w. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna virus in the brain. Science. 1983 Jun 24;220(4604):1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983 Aug;148(2):305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Price R. W., Brew B. J., Rosenblum M. The AIDS dementia complex and HIV-1 brain infection: a pathogenetic model of virus-immune interaction. Res Publ Assoc Res Nerv Ment Dis. 1990;68:269–290. [PubMed] [Google Scholar]
- Quagliarello V. J., Wispelwey B., Long W. J., Jr, Scheld W. M. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Invest. 1991 Apr;87(4):1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244–4250. [PubMed] [Google Scholar]
- Richt J., Stitz L., Deschl U., Frese K., Rott R. Borna disease virus-induced meningoencephalomyelitis caused by a virus-specific CD4+ T cell-mediated immune reaction. J Gen Virol. 1990 Nov;71(Pt 11):2565–2573. doi: 10.1099/0022-1317-71-11-2565. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar V., Kao M., Hamir A. N., Sheng H., Koprowski H., Dietzschold B. Kinetics of virus spread and changes in levels of several cytokine mRNAs in the brain after intranasal infection of rats with Borna disease virus. J Virol. 1992 Feb;66(2):992–998. doi: 10.1128/jvi.66.2.992-998.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R. D., Willenborg D. O. Direct injection of cytokines into the spinal cord causes autoimmune encephalomyelitis-like inflammation. J Neurol Sci. 1990 Dec;100(1-2):37–42. doi: 10.1016/0022-510x(90)90010-k. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stitz L., Sobbe M., Bilzer T. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J Virol. 1992 Jun;66(6):3316–3323. doi: 10.1128/jvi.66.6.3316-3323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Brady L. S., Smith M. A., Mamalaki E., Fox R. J., Herkenham M. Optimization of cRNA probe in situ hybridization methodology for localization of glucocorticoid receptor mRNA in rat brain: a detailed protocol. Cell Mol Neurobiol. 1990 Mar;10(1):145–157. doi: 10.1007/BF00733641. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Zentel H. J., Nohr D., Müller S., Yanaihara N., Weihe E. Differential occurrence and distribution of galanin in adrenal nerve fibres and medullary cells in rodent and avian species. Neurosci Lett. 1990 Dec 11;120(2):167–170. doi: 10.1016/0304-3940(90)90029-9. [DOI] [PubMed] [Google Scholar]
- Zentel H. J., Weihe E. The neuro-B cell link of peptidergic innervation in the Bursa Fabricii. Brain Behav Immun. 1991 Mar;5(1):132–147. doi: 10.1016/0889-1591(91)90012-y. [DOI] [PubMed] [Google Scholar]