Abstract
Collectively, the xanthophyll class of carotenoids perform a variety of critical roles in light harvesting antenna assembly and function. The xanthophyll composition of higher plant photosystems (lutein, violaxanthin, and neoxanthin) is remarkably conserved, suggesting important functional roles for each. We have taken a molecular genetic approach in Arabidopsis toward defining the respective roles of individual xanthophylls in vivo by using a series of mutant lines that selectively eliminate and substitute a range of xanthophylls. The mutations, lut1 and lut2 (lut = lutein deficient), disrupt lutein biosynthesis. In lut2, lutein is replaced mainly by a stoichiometric increase in violaxanthin and antheraxanthin. A third mutant, aba1, accumulates normal levels of lutein and substitutes zeaxanthin for violaxanthin and neoxanthin. The lut2aba1 double mutant completely lacks lutein, violaxanthin, and neoxanthin and instead accumulates zeaxanthin. All mutants were viable in soil and had chlorophyll a/b ratios ranging from 2.9 to 3.5 and near wild-type rates of photosynthesis. However, mutants accumulating zeaxanthin exhibited a delayed greening virescent phenotype, which was most severe and often lethal when zeaxanthin was the only xanthophyll present. Chlorophyll fluorescence quenching kinetics indicated that both zeaxanthin and lutein contribute to nonphotochemical quenching; specifically, lutein contributes, directly or indirectly, to the rapid rise of nonphotochemical quenching. The results suggest that the normal complement of xanthophylls, while not essential, is required for optimal assembly and function of the light harvesting antenna in higher plants.
The xanthophyll class of carotenoids are oxygenated derivatives of α and β-carotene (Fig. 1A). Xanthophylls are present in all photosynthetic organisms and contribute to light harvesting, photoprotection, and assembly of the light harvesting antenna complexes (LHC). Over 600 different naturally occurring carotenoids have been identified to date. This remarkable diversity is reflected in the composition of the photosynthetic apparatus of different bacterial and algal classes. However, the xanthophyll composition of the photosystems in green algae and plants is remarkably conserved. Typically, four carotenoids accumulate to substantial levels in photosystems across the vast majority of green plant species, including angiosperms, gymnosperms, green algae, pteridophytes, and bryophytes (1). Lutein is the most abundant (up to 50% of total), with β-carotene, violaxanthin and neoxanthin occurring in decreasing abundance (Fig. 1B). β-carotene is essential for the assembly and photoprotection of photosystem II reaction centers. The xanthophylls, lutein, violaxanthin, and neoxanthin, are enriched in the LHCs, where they contribute to assembly, light harvesting, and photoprotection (2–8).
Figure 1.
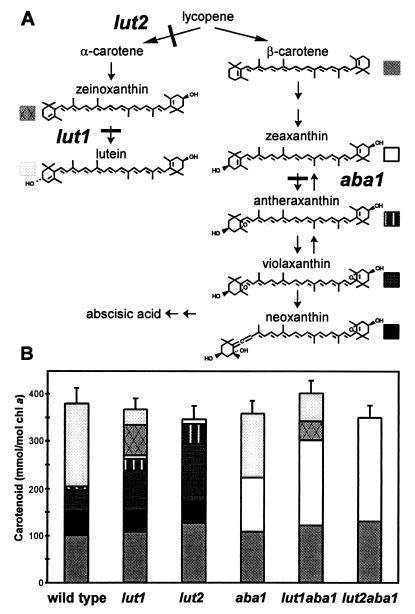
Carotenoid biosynthetic pathway in higher plant chloroplasts commencing with lycopene (A). lut1, lut2, and aba1 mutations are shown. Carotenoid content of mature green leaves of wild-type and xanthophyll mutant lines are shown in the bar graph (B). Each section of the bar corresponds to a specific carotenoid of the pathway (A). The SDs of the total pool of carotenoids per mole chl a are shown.
A summary of the carotenoid biosynthetic pathway of higher plants and relevant chemical structures is shown in Fig. 1. Lycopene is cyclized twice by the enzyme lycopene β-cyclase to form β-carotene. The two beta rings of β-carotene are subjected to identical hydroxylation reactions to yield zeaxanthin, which in turn is epoxidated once to form antheraxanthin and twice to form violaxanthin. Neoxanthin is derived from violaxanthin by an additional rearrangement (9). The reversible interconversion of violaxanthin to zeaxanthin (the xanthophyll cycle) is a set of light-dependent reactions that are involved in acclimation to light stress (10). Although all photosynthetic organisms contain β-carotene derivatives, higher plants and green algae have an additional unique class of carotenoids, the α-carotene derivatives (β,ɛ-carotenoids), which are derived from lycopene by the action of two structurally related enzymes, lycopene β-cyclase and lycopene ɛ-cyclase (11). Hydroxylation of the β ring of α-carotene forms zeinoxanthin and subsequent hydroxylation of the ɛ ring forms lutein (12).
During photomorphogenesis, a massive synthesis of photosystem proteins, lipids, and pigments is coupled with their coordinated assembly into functional photosystems. The minor LHC apoproteins probably first associate with the photosystem II core complexes (CP43, CP47, D1/2), then monomers of LHCIIb (Lhcb1/2) form trimers (13) and associate with the growing complex (14). Both chlorophylls and carotenoids are required for proper folding, assembly, and stability of LHC apoproteins (15, 16), as demonstrated by detailed in vitro reconstitution studies of pigment–protein complexes (16–19). Maximum reconstitution efficiency and stability of LHCIIb complexes was achieved when chlorophyll (chl) a, chl b, and the three normal leaf xanthophylls (lutein, violaxanthin, and neoxanthin) were used. Any two of the three xanthophylls could support in vitro reconstitution to varying degrees (17), but when a single xanthophyll was used, only lutein enabled limited assembly. Although this approach has provided considerable insight into the minimal structural requirements for the assembly process of an individual LHC in vitro, it cannot be used to elucidate the roles of each xanthophyll in higher order LHC structures and the complex processes of light harvesting and photoprotection. Also, as with all in vitro studies, it is necessary to confirm such results in vivo.
High light stress results in multiple changes to the photosynthetic apparatus, including state transitions (20) and a decrease in LHCII trimers and the photosystem II/photosystem I ratio (21), which cumulatively provide enhanced photoprotection. Among the most rapid of these responses is the induction of the xanthophyll cycle (10) and nonphotochemical quenching (NPQ) in the LHCs. Physiological (22–24) and genetic (25, 26) studies have demonstrated the important role of zeaxanthin synthesis via the xanthophyll cycle in NPQ. Elegant studies on the photophysical properties of the carotenoids and chlorophylls as they relate to light harvesting, energy transfer, and photoprotection have provided insights into the underlying mechanisms involved (27–30). The photophysical data suggest that violaxanthin can function in light harvesting, transferring energy to chlorophyll, whereas zeaxanthin and β-carotene are capable of acting as energy acceptors, thereby quenching excited chlorophyll molecules. It is difficult to draw a clear conclusion for lutein, because the S1 excited energy state of lutein is predicted to be very similar to the Qy excited state of chl a and b. Transient absorbance studies of energy transfer from carotenoids to chlorophylls have implicated lutein in light harvesting (31). Though it is assumed that lutein acts as a light harvesting pigment, its actual role in vivo has yet to be rigorously defined.
Lutein is the most abundant carotenoid in all photosynthetic plant tissues, and its synthesis and presence are evolutionarily conserved both in land plants and green algae. The apparent localization of lutein in crystallized LHCIIb (32) and its requirement for optimal in vitro assembly of LHCs (16–19) had led to the assumption that lutein is critical for higher plant photosystem assembly and function. Somewhat surprisingly, Arabidopsis lutein-deficient lut mutants were viable (12, 30). The lut2 mutation eliminates lutein production and is biochemically and genetically consistent with a disruption in the gene encoding the enzyme lycopene ɛ-cyclase (Fig. 1; ref. 12). The lut1 mutation results in the accumulation of the precursor of lutein, zeinoxanthin, and is consistent with disruption of the gene encoding the ɛ-ring hydroxylase enzyme. In nature, a few examples of substitutions for lutein have been observed: lactuxanthin for a part of the lutein pool in lettuce (33) and violaxanthin for lutein in an Eustigmatophyte alga that lacks chl b, Nannochloropsis salina (34, 35). Presumably, lut mutant viability is caused by partial or total functional compensation by other xanthophylls, in particular violaxanthin and antheraxanthin, which increase significantly in the absence of lutein (12).
Analogous mutations affecting lutein production exist in green algae; however, there seems to be significant differences in the effects of xanthophyll deficiency in these organisms. In a mutant of the green alga Scenedesmus obliquus, the absence of lutein resulted in a reduction in total chlorophyll, a decrease in chl b (the chl a/b ratio was 6.54 vs. 3.06 for the wild type) (36, 37), and the near complete absence of the major LHCII proteins, although minor LHCII proteins were largely unaffected (38). Though the genetic basis of this Scenedesmus mutant is not known, it is likely to be an orthologue of the lut2 locus, that is, a disruption of the lycopene ɛ-cyclase gene. Another putative orthologous mutant to lut2 is the lor1 mutation in the green alga Chlamydomonas (39). The pleiotropic effects of this mutation appear to be intermediate between the putative orthologues of Scenedesmus and Arabidopsis with a chl a/b ratio of 4.02 vs. 2.64 in the wild type (25). Clearly, although lutein synthesis is evolutionarily conserved in green algae and plants, there are marked differences in the effects of lutein deficiency on LHC pigment content, assembly, and function in these organisms. This suggests that either the functional roles of lutein or the degree of functional plasticity of different xanthophylls differ somewhat between plants and algae.
These observations led us to consider the limits to plasticity in carotenoid substitutions that would still enable viable assembly, light harvesting, and photoprotection in higher plant photosystems in vivo and in this way specifically address the question of why the three xanthophylls, lutein, violaxanthin, and neoxanthin, are so highly conserved across the higher plant species. In this study, we examined a series of single and double xanthophyll biosynthetic mutants of Arabidopsis that allow the selective and specific elimination of whole classes of xanthophylls, often replacing them with xanthophylls that normally occur only at low levels or, in some cases, not at all in higher plant photosystems. The mutations used were the lutein-deficient lines lut1 and lut2 and the neoxanthin and violaxanthin-deficient line aba1 (12, 40, 41). The consequences of modified xanthophyll compositions in single and double mutants in relation to photomorphogenesis and energy transfer processes under low and high light have been investigated.
MATERIALS AND METHODS
Growth of Plants.
Arabidopsis thaliana wild-type ecotypes Columbia (Col-4) and Landsberg erecta (Ler-O) and xanthophyll mutants were grown in soil under 12 h/day light (≈140 μmol⋅m−2⋅sec−1). In all experiments, no difference was observed between the two wild-type ecotypes. The lut1–1 and lut2–1 mutants were back-crossed to Col-4 four times and then were crossed with aba1–3 (Ler), which was obtained from the Arabidopsis Biological Resource Center (Ohio State Univ., Columbus, OH). The double lut2aba1 and lut1aba1 F2 mutants were selected by HPLC.
For determination of the rate of greening, seed were plated on sterile media (42) containing 3% sucrose, were vernalized for 4 days at 4°C, were etiolated for 3 days at 20°C, and then were transferred to photosynthetically active radiation of 70 μmol⋅m−2 sec−1. A separate set of plates were treated with 2 ml of 5 μM abscisic acid (ABA) before exposure to light. Seedlings (cotyledons, leaves, and 2–7 mm of hypocotyl) were harvested at regular intervals, were pooled in two replicates of ten seedlings, and were frozen in liquid nitrogen.
Pigment Analysis.
Green leaf tissue of 3- to 6-week-old plants was harvested and was frozen immediately in liquid nitrogen for pigment analysis. Leaf tissue (20–100 mg) was extracted in 500 μl of N′N′-dimethylformamide for 3 h in the dark. Chlorophyll content was determined by the method of Porra et al. (43) and by HPLC. N′N′-dimethylformamide extract (40 μl) was fractionated by HPLC (Hewlett–Packard series 1100) as described (42). Pigments were identified by retention time and spectrophotometric properties by using a photodiode-array detector and were quantified by integrating peak areas (12). The correlation coefficient (R2) of the standard curves was >99.40 and was linear from 20 to 8000 ng.
Chlorophyll Fluorescence.
Chlorophyll fluorescence measurements were performed as described (26). Standard fluorescence nomenclature was used (44). NPQ is defined as (Fm–Fm′)/Fm′, where Fm is defined as the maximum fluorescence in the dark adapted state and Fm′ is the maximum fluorescence in any light-adapted state.
RESULTS
Pigment Composition and Viability of Xanthophyll Mutants.
Fig. 1A shows the major intermediates and steps of the carotenoid biosynthetic pathway in higher plants and blocks in the pathway caused by the mutations lut1, lut2, and aba1. The level and composition of carotenoids in the single mutant lines were consistent with previous reports (12, 40, 41, 45), as shown in Fig. 1B. Missing carotenoids were replaced by an equimolar increase in other specific carotenoids such that there was no little variation in total carotenoid content (per mole of chl a or per gram of fresh weight). In particular, the precursor of the site of blockage accumulated in aba1 (zeaxanthin) and lut1 (zeinoxanthin), and, in lut1 and lut2, there was a specific increase in violaxanthin and antheraxanthin. There was little, if any, change in β-carotene content in the three single mutant lines. The carotenoid composition of the double mutants was as predicted from the single mutant phenotypes (Fig. 1B). The lut2aba1 double mutant accumulated zeaxanthin and β-carotene albeit at slightly lower levels than the wild type whereas lut1aba1 accumulated zeaxanthin and β-carotene as the principal carotenoids as well as smaller amounts of zeinoxanthin and lutein.
Significantly, xanthophyll mutants that accumulated zeaxanthin were virescent; that is, they exhibited delayed greening during photomorphogenesis because of slower rates of chlorophyll accumulation (Fig. 2 A and B). The virescence phenotype in aba1, lut1aba, and lut2aba1 was observed in seedlings grown on soil or agar medium, with or without a prior etiolation treatment (Fig. 2 A and B). The most severe phenotype was observed in lut2aba1, in which zeaxanthin was the only xanthophyll. In fact, the condition was semilethal; that is, ≈30% of the seedlings germinated in soil did not survive. When germinated on sucrose media, lut2aba1 seedlings were virescent and 100% viable if transferred to soil after the seedlings were green. However, if the transfer to soil occurred before the seedlings had greened fully, there was still a high mortality rate. The rate of greening for aba1 and lut1aba1 was intermediate to the wild type and lut2aba1, with lut1aba1 often a little quicker to green (the rate of greening was wild type > lut1aba1 ≥ aba1 > lut2aba1; Fig. 2 and results not shown). Yet, there was no difference between the single lut mutants and the wild type in the rate of greening.
Figure 2.
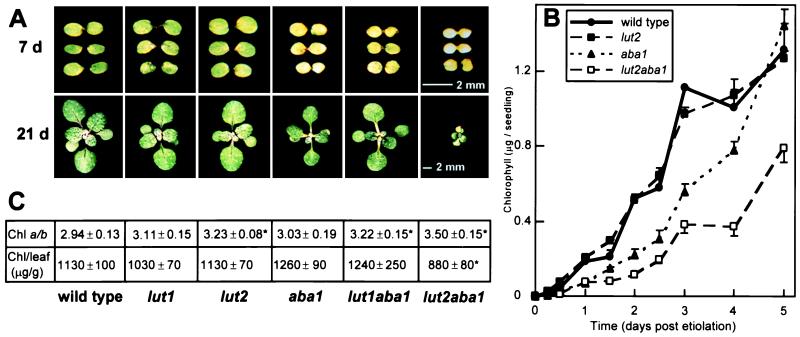
Photographs of developing seedlings (A), rate of chlorophyll accumulation in etiolated seedlings (B), and chlorophyll content of mature green leaves (C) of wild-type and mutant lines. (A) Photographs of soil-grown wild-type and xanthophyll mutant seedlings at 7 and 21 days, grown on a 12-h light cycle. The scale for all 7- and 21-day seedlings are shown by bars in the 7- and 21-day lut2aba1 panels, respectively. (B) Rate of total chlorophyll accumulation in seedlings. Wild-type, lut2, aba1, and lut2aba1 etiolated tissue culture-grown seedlings were vernalized, were germinated in the dark for 3 days, and then were transferred to continuous light. At each time point, two replicate extracts of 10 pooled seedlings were analyzed, and the chlorophyll content was expressed on a per plant basis (micrograms per seedling). SDs greater than the size of the symbol are shown. Note that the rate of greening is slower for 12-h-day soil-grown seedlings than for 24-h-day tissue culture-grown seedlings. (C) Chlorophyll ratios and content of mature green leaves. The chl a/b ratio (mol/mol) and total chlorophyll content of green leaves (micrograms per gram fresh weight) with SDs are shown. Values that are significantly different (P < 0.05) from the wild type are marked with an asterisk (∗).
To address whether the virescence phenotype in aba1 and the lutaba double mutants was a response to ABA deficiency or modification of xanthophyll compositions, we tested the effects of exogenous ABA on greening of etiolated seedlings. There was no significant effect on the rate of chlorophyll accumulation in any line in the presence of ABA after 1 day; the response was more variable after 4 days, but no significant trends were detectable (results not shown). Furthermore, we used the aba1–3 allele because, compared with other aba1 alleles, it produces more ABA and exhibits nearly wild-type size, water relations, and vigor (40). From these results, we consider that the delay in greening is likely caused by alterations in xanthophyll content.
Eventually, aba1 and lut1aba1 seedlings accumulated substantial quantities of chlorophyll, reaching wild-type levels on a fresh weight basis (Fig. 2C). However, in lut2aba1 the plants were much smaller than the wild type throughout their development (Fig. 2A), the amount of chlorophyll was slightly lower, and the chl a/b ratio was higher (3.5 vs. 2.94) than the wild type (Fig. 2C). In our previous study, we did not detect a significant change in chlorophyll content or the chlorophyll a/b ratio in lut1 and lut2 (12). When these analyses were repeated in this study with a larger number of replicates and a more refined protocol, such that minor differences could be detected, the chl a/b ratio of lut2 was slightly but significantly higher than the wild type (3.23 vs. 2.94), as were lut1aba1 (3.22) and lut2aba1 (3.5) (Fig. 2C).
Nonphotochemical Quenching and Photosynthetic Rates.
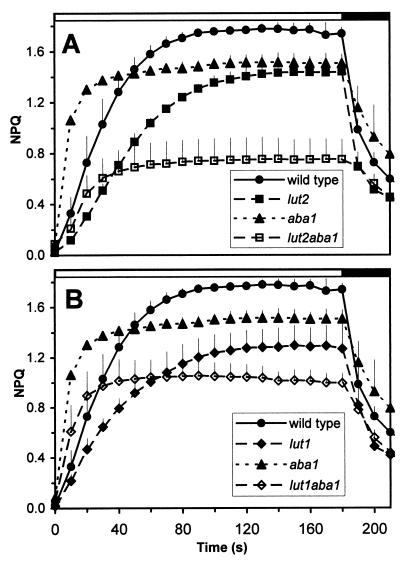
We used the mutants to investigate the effects of altered xanthophyll compositions on the magnitude and timing of NPQ of chlorophyll fluorescence during exposure to high light. In wild-type plants, the NPQ response was rapid, reaching a plateau after 80 sec of illumination with high light (Fig. 3). However, in the lut2 and lut1 mutants, which are defective in lutein synthesis, the induction of NPQ was inhibited significantly. After 10 sec of illumination, the level of NPQ in lut2 was only one-third that of the wild type, and 120 sec were required before NPQ plateaued. The NPQ maxima was often lower in lut1 and lut2 than in the wild type, despite a much larger zeaxanthin pool in high light-treated lut1 and lut2 plants (results not shown). The patterns of NPQ induction and maximal NPQ levels were similar for lut1 and lut2 (compare Fig. 3 A and B). In contrast, the aba1 mutant, which has lutein and zeaxanthin as its xanthophylls, had a greater rate of NPQ induction, being 3-fold higher at 10 sec than the wild type. The maximum level of NPQ in aba1 appeared lower than in wild type, because aba1 had a 30% lower Fm, resulting in a lower calculated NPQ. In the lut1aba1 and lut2aba1 double mutants, high constitutive levels of zeaxanthin restored the rapid phase of NPQ that was defective in the lutein-deficient single mutants. However, the maximal level of NPQ in these double mutants was much lower than in the wild type or in any of the single mutants; whether this can be explained solely by a lower Fm or whether it also reflects destabilized LHCs or reduced availability of zeaxanthin is not known.
Figure 3.
Induction of nonphotochemical quenching (NPQ) in wild-type and xanthophyll mutant lines in the lut2 background (A) and the lut1 background (B). Plants were grown at 140 μmol⋅m−2⋅sec−1 for 12-h cycles and were dark adapted overnight. Fluorescence was measured before, during, and after exposure to actinic light (photosynthetically active radiation of 1,083 μmol⋅m−2⋅sec−1), shown by the white bar above the graphs; the dark bar indicates illumination with a weak, far-red background light. Three replicate plants were analyzed for each line and were averaged. SDs greater than the size of the symbol are shown.
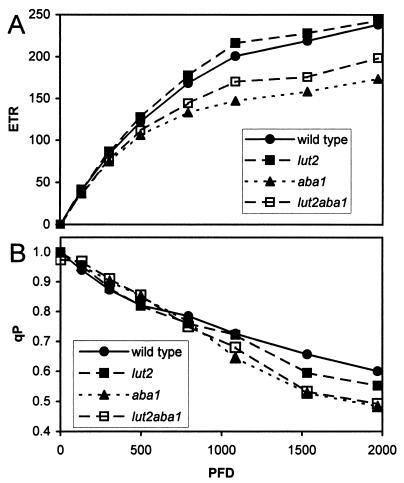
Fig. 4A demonstrates that mutant and wild-type plants had the same electron transport rate (ETR) for light intensities up to 500 μmol⋅m−2⋅sec−1. Thus, the ETR of all strains would be indistinguishable under the growth conditions that are used routinely for Arabidopsis (70–300 μmol⋅m−2 sec−1). At higher intensities, the ETR for lut2 and wild-type plants were almost identical but were 25% lower for aba1 and lut2aba1. The oxidation state of QA, as estimated by the extent of photochemical quenching (qP) of chlorophyll fluorescence, was 1 in the dark and declined almost linearly for all lines (Fig. 4B). The qP for all strains was indistinguishable from 0 to 1,000 μmol⋅m−2 sec−1. At higher intensities, the level of qP varied slightly (wt > lut2 > aba1 = lut2aba1).
Figure 4.
Light response curves of wild-type, lut2, aba1, and lut2aba1 lines were used to calculate the electron transport rate, ETR (A), and photochemical quenching, qP (B). The photosynthetic activity radiation intensities of 0, 132, 304, 499, 794, 1,087, 1,533, and 1,971 μmol⋅m−2⋅sec−1 were applied for 10 min. The fluorescence measurements, Fs and Fm′ values (light), and the Fo′ value (recorded in dark immediately after each step) were used to calculate the ETR and qP.
DISCUSSION
The xanthophylls, lutein, neoxanthin, and violaxanthin, are required for in vitro reconstitution of LHC complexes, and their near ubiquitous presence throughout the higher plant kingdom indicates their collective importance in growth and development. However, the respective roles of each xanthophyll in assembly, light harvesting, and photoprotection are poorly defined. Consequently, we have investigated the roles of different xanthophylls in vivo by selectively eliminating and substituting the xanthophylls by using the lut1, lut2, and aba1 mutations of Arabidopsis. The first and most important conclusion is that plants are viable in the complete absence of all three major xanthophylls (lutein, violaxanthin, and neoxanthin). In the most extreme case, we have generated viable plants in which the only carotenoids are β-carotene and zeaxanthin. An analogous Scenedesmus mutant is also viable, albeit somewhat impaired (37). In Arabidopsis lut2aba1, under normal growth conditions, the level of chlorophyll per unit of tissue, the chlorophyll a/b ratio, and the photosynthetic rate, although altered, were still remarkably similar to the wild type. However, the story is not that simple because a detailed analysis of growth and development revealed that the three major xanthophylls are required for optimal rates of seedling development and photoprotection.
The rate of greening is severely impaired in aba1, lut1aba1, and lut2aba1 seedlings that accumulate substantial quantities of zeaxanthin at the expense of neoxanthin and violaxanthin (aba1) or all three xanthophylls (lut1aba1 and lut2aba1). The most severe phenotype was the lut2aba1 background in which there were no wild-type xanthophylls, only zeaxanthin. Chlorophyll accumulation and, by inference, antenna assembly were delayed in lut2aba1, and many seedlings were not viable (Fig. 2A). Yet, most of the chlorotic seedlings eventually greened and were fully viable, although the resulting plants were much smaller and consequently set fewer seed than wild-type plants. This was not caused by any difference in photosynthetic capacity of the mature plant (Fig. 4) but was the result of the delayed growth during the early stages of development. The capacity to green eventually despite the initial chlorosis indicates an inherent capability to adjust to suboptimal pigment stoichiometries by an undefined compensatory mechanism.
The virescence of aba1, lut1aba1, and lut2aba1 seedlings may be caused by an inability of specific LHC apoproteins to bind zeaxanthin with the subsequent greening because of an increase in abundance of another LHC apoprotein that is capable of incorporating zeaxanthin. Some, but not all, reports have shown a preferential binding of xanthophyll cycle pigments by minor LHCs compared with LHCIIb (3, 46). An alternative thesis is that zeaxanthin competitively inhibits antenna formation. That is, zeaxanthin may bind with the apoproteins but may do so in such a manner that the complexes are poorly formed. In support of these theses, aba1 plants have a pronounced decrease in LHCIIb trimers in preference for monomers and an increase in free zeaxanthin and chl b (47).
Zeaxanthin and lutein are very similar molecules (Fig. 1A). Consequently, it may seem surprising that they could differ to a large extent in their ability to bind with LHC apoproteins. However, there are two important structural differences between zeaxanthin and lutein. First, the chirality of the two hydroxyl groups is the same for zeaxanthin and opposing for lutein. Second, the positioning of the double bond in the epsilon ring alters the three-dimensional shape such that it lies at a different angle to the β ring and the conjugated backbone. The angle of the epsilon ring of lutein is the same as that for the epoxy ring(s) of antheraxanthin and violaxanthin, which seem to substitute effectively for lutein (Fig. 2). Therefore, the three-dimensional shape of the xanthophylls is apparently important in pigment–protein interactions, and the planer zeaxanthin may be incorporated less effectively.
The photosynthetic efficiency of mature plants was surprisingly unaffected by any of the xanthophyll compositions at moderate light intensities. This demonstrates that, once green, the antenna can assemble, capture, and transfer light energy efficiently with a range of xanthophyll compositions, including zeaxanthin. In fact, zeaxanthin to chl a energy transfer in isolated LHCs from aba1 has been observed (31). The reduction in photosynthetic capacity under high light for aba1 plants was not observed by others under their growth conditions (47, 48) and consequently should be interpreted with some caution. Reduced ETRs may reflect more photodamage at high light in lut2aba1 plants, resulting in reduced photosynthetic capacity. Therefore, the accumulation of a photoprotective xanthophyll, zeaxanthin, may still result in increased damage if other variables, such as photosystem assembly and stability, are compromised.
The widely held view of the xanthophyll cycle is that zeaxanthin and antheraxanthin are the only carotenoids that contribute to NPQ (10, 22, 49). In complete contrast, we have shown that the Arabidopsis lutein mutants have both delayed and reduced levels of NPQ (Fig. 3). Likewise, a reduction in the level of NPQ also was obtained in the lor1 mutant of Chlamydomonas (25), which lacks lutein. The exact molecular basis of Chlamydomonas lor1 is not defined, and it has a chl a/b ratio of 4.02 vs. 2.64 for the wild type (25), which may indicate a destabilized antenna. Consequently, the question arose whether the loss in NPQ in lor1 was caused by an indirect effect of lutein deficiency on the state of the antenna. In Arabidopsis lut mutants, the absence of lutein had little or no effect on chl a/b ratios (Fig. 2C), implying less disruption to the antenna because of the substitution of lutein by other xanthophylls; however, NPQ was still delayed and reduced (Fig. 3). Therefore, the genetic analysis in both Chlamydomonas and Arabidopsis suggests that, in addition to zeaxanthin and antheraxanthin, lutein contributes to NPQ either directly or indirectly (Fig. 3; refs. 25 and 50). Specifically, lutein appears to be involved in the rapid induction of NPQ, which was delayed markedly in the lut mutants on illumination with high light. The reduction in NPQ in the lor1 and lut mutants was seen despite increased levels of violaxanthin and antheraxanthin. Violaxanthin would not be expected to quench (29). However, antheraxanthin is a photophysical homologue of lutein in vitro (29) and can contribute to quenching (22–24, 51). Consequently, it is surprising that, if lutein (wild type) and zeaxanthin (aba1, lut2aba1) are abundant, quenching is rapid but is not so if antheraxanthin is abundant (lut2). It is possible that the accumulated antheraxanthin in lut2 and lut1 is unavailable to the site of NPQ. The NPQ induction curves for lut2 and lut1 are impeded similarly despite the fact that lutein content varies from 0 to 20% of wild-type levels, which suggests that not all lutein molecules can impact on NPQ. Therefore, only a part of the pool of the quenching xanthophylls are required and can alter NPQ. The conservation of lutein’s augmentation of NPQ in Arabidopsis and Chlamydomonas implies that not only is lutein ubiquitous in green algae and higher plants, so may be its contribution to NPQ.
The question remains as to whether lutein directly or indirectly impacts NPQ. The argument for an indirect contribution would be that the absence of lutein alters LHC antenna structure to impede quenching. The LHC is protonated during light stress, which is hypothesized to result in conformation change promoting NPQ (52). It may be that the loss of lutein from the antenna deleteriously affects this protonation-induced conformation change and thereby reduces quenching. The alternative thesis is that lutein directly contributes to fluorescence quenching, which necessitates that lutein has both the photophysical capacity and correct location to quench singlet chlorophyll. The predicted excited S1 energy state of lutein has a spectral overlap with the excited states of chlorophyll, which would enable it to act as a quencher (29). When considering the mechanism of lutein-induced quenching, it should be noted that the constitutive presence of zeaxanthin (but not violaxanthin or antheraxanthin) complemented the role of lutein in the rapid phase of NPQ (Fig. 3). This is despite the fact that zeaxanthin accumulation had the most deleterious effects on LHC structure as inferred by delayed greening. The presence of a quenching carotenoid before light stress results in faster NPQ induction, presumably because NPQ then depends solely on build-up of a large transmembrane proton gradient on illumination. There are valid arguments for both theses, so, clearly, more work is required to define whether lutein directly or indirectly contributes to quenching in vivo.
The plasticity of xanthophyll substitutions in photosystem assembly considered together with the conservation of xanthophyll composition across higher plant species presents a paradox. Our genetic analyses reveals a solution to the paradox: A degree of functional redundancy enhances the chances of survival if one component is lost; however, plant fitness and plasticity are optimized by the presence of all wild-type xanthophylls because of the distinct functions of each. For example, the involvement of related xanthophylls (lutein, zeaxanthin, and antheraxanthin) in NPQ provides some functional redundancy, but the presence of all quenching xanthophylls is necessary for maximal NPQ, which can enhance plant viability (Fig. 3; ref. 25). Likewise, mutations substituting lutein, violaxanthin, and antheraxanthin result in viable plants in a controlled environments, although overall fitness is compromised by aberrant xanthophyll profiles. This was clearly the case for the lut2aba1 mutant, which exhibited reduced seedling viability, smaller plants, and, consequently, fewer seed. Therefore, although the structural and photophysical similarities of the xanthophylls provide a degree of functional plasticity, optimal fitness is achieved by maintaining the wild-type complement of xanthophylls.
ABBREVIATIONS
- ABA
abscisic acid
- chl
chlorophyll
- ETR
electron transport rate
- LHC
light harvesting complex
- NPQ
nonphotochemical quenching
- qP
photochemical quenching of chlorophyll fluorescence
References
- 1. Strain H. In: Biochemistry of Chloroplasts. Goodwin T, editor. I. New York: Academic; 1966. pp. 387–406. [Google Scholar]
- 2.Grossman A R, Bhaya D, Apt K E, Kehoe D M. Annu Rev Genet. 1995;29:231–288. doi: 10.1146/annurev.ge.29.120195.001311. [DOI] [PubMed] [Google Scholar]
- 3.Peter G F, Thornber J P. J Biol Chem. 1991;266:16745–16754. [PubMed] [Google Scholar]
- 4.Green B R, Durnford D G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:685–714. doi: 10.1146/annurev.arplant.47.1.685. [DOI] [PubMed] [Google Scholar]
- 5.Dreyfuss B W, Thornber J P. Plant Physiol. 1994;106:829–839. doi: 10.1104/pp.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank H, Cogdell R J. In: Carotenoids in Photosynthesis. Young A J, Britton G, editors. London: Chapman & Hall; 1993. pp. 253–326. [Google Scholar]
- 7.Siefermann-Harms D. Physiol Plant. 1987;69:561–568. [Google Scholar]
- 8.Horton P, Ruban A V, Walters R G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 9.Britton G. In: Plant Pigments. Goodwin T W, editor. San Diego: Academic; 1988. pp. 133–182. [Google Scholar]
- 10.Pfundel E, Bilger W. Photosynth Res. 1994;42:89–109. doi: 10.1007/BF02187121. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham F X, Pogson B J, Sun Z, McDonald K, DellaPenna D, Gantt E. Plant Cell. 1996;8:1613–1626. doi: 10.1105/tpc.8.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogson B, McDonald K, Truong M, Britton G, DellaPenna D. Plant Cell. 1996;8:1627–1639. doi: 10.1105/tpc.8.9.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansson S. Biochim Biophys Acta. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 14.Dreyfuss B W, Thornber J P. Plant Physiol. 1994;106:841–848. doi: 10.1104/pp.106.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbeck K, Romer S, Senger H. Planta. 1989;179:242–250. doi: 10.1007/BF00393695. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen H, Finkenzeller B, Kuhlein N. Eur J Biochem. 1993;215:809–816. doi: 10.1111/j.1432-1033.1993.tb18096.x. [DOI] [PubMed] [Google Scholar]
- 17.Plumley F G, Schmidt G W. Proc Natl Acad Sci USA. 1987;84:146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulsen H, Rumler U, Rudiger W. Planta. 1990;181:204–211. doi: 10.1007/BF02411539. [DOI] [PubMed] [Google Scholar]
- 19.Cammarata K V, Plumley F G, Schmidt G F. In: Current Research in Photosynthesis. Baltscheffsky M, editor. Vol. 2. Dordrecht, The Netherlands: Kluwer; 1990. pp. 341–344. [Google Scholar]
- 20.Gal A, Zer H, Ohad I. Physiol Plant. 1997;100:869–885. [Google Scholar]
- 21.Walters R G, Horton P. Planta. 1994;195:248–256. [Google Scholar]
- 22.Demmig-Adams B, Adams W W., III Planta. 1996;198:460–470. [Google Scholar]
- 23.Demmig-Adams B, Adams W W., III Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- 24.Gilmore A M, Yamamoto H Y. Photosynth Res. 1993;35:67–78. doi: 10.1007/BF02185412. [DOI] [PubMed] [Google Scholar]
- 25.Niyogi K, Björkman O, Grossman A. Proc Natl Acad Sci USA. 1997;94:14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niyogi K, Grossman A, Björkman O. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shreve A P, Trautman J K, Frank H A, Owens T G, Albrecht A C. Biochim Biophys Acta. 1991;1058:280–288. doi: 10.1016/s0005-2728(05)80248-8. [DOI] [PubMed] [Google Scholar]
- 28.Trautman J K, Shreve A P, Violette C A, Frank H A, Owens T G, Albrecht A C. Proc Natl Acad Sci USA. 1990;87:215–219. doi: 10.1073/pnas.87.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank H A, Cua A, Chynwat V, Young A, Gosztola D, Wasielewski M R. Photosynth Res. 1994;41:389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- 30.Frank H A, Cogdell R J. Photochem Photobiol. 1996;63:257–264. doi: 10.1111/j.1751-1097.1996.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 31.Connelly J P, Mueller M G, Bassi R, Croce R, Holzwarth A R. Biochemistry. 1997;36:281–287. doi: 10.1021/bi962467l. [DOI] [PubMed] [Google Scholar]
- 32.Kuhlbrandt W, Wang D N, Fujiyoshi Y. Nature (London) 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- 33.Phillip D, Young A J. Photosynth Res. 1995;43:273–282. doi: 10.1007/BF00029940. [DOI] [PubMed] [Google Scholar]
- 34.Sukenik A, Livne A, Neori A, Yacobi Y Z, Katcoff D. Plant Cell Physiol. 1992;33:1041–1048. [Google Scholar]
- 35.Brown J S. Plant Physiol. 1987;83:434–437. doi: 10.1104/pp.83.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishop N I, Urbig T, Senger H. FEBS Lett. 1995;367:158–162. doi: 10.1016/0014-5793(95)00510-g. [DOI] [PubMed] [Google Scholar]
- 37.Bishop N I. J Photochem Photobiol B. 1996;36:279–283. [Google Scholar]
- 38.Heinze I, Pfuendel E, Huehn M, Dau H. Biochim Biophys Acta. 1997;1320:188–194. [Google Scholar]
- 39.Chunaev A S, Mirnaya O N, Maslov V G, Boschetti A. Photosynthetica. 1991;25:291–301. [Google Scholar]
- 40.Rock C D, Zeevaart J A D. Proc Natl Acad Sci USA. 1991;88:7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duckham S C, Linforth R S T, Taylor I B. Plant Cell Environ. 1991;14:601–606. [Google Scholar]
- 42.Norris S R, Barrette T R, DellaPenna D. Plant Cell. 1995;7:2139–2149. doi: 10.1105/tpc.7.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porra R J, Thompson W A, Kriedemann P E. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- 44.Van Kooten O, Snel J. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- 45.Pogson B, Norris S, McDonald K, Truong M, DellaPenna D. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. IV. Dordrecht, The Netherlands: Kluwer; 1995. pp. 75–78. [Google Scholar]
- 46.Bassi R, Pineau B, Dainese P, Marquardt J. Eur J Biochem. 1993;212:297–303. doi: 10.1111/j.1432-1033.1993.tb17662.x. [DOI] [PubMed] [Google Scholar]
- 47.Tardy F, Havaux M. J Photochem Photobiol. 1996;34:87–94. doi: 10.1016/1011-1344(95)07272-1. [DOI] [PubMed] [Google Scholar]
- 48.Hurry V, Anderson J M, Chow W S, Osmond C B. Plant Physiol. 1997;113:639–648. doi: 10.1104/pp.113.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilmore A M. Physiol Plant. 1997;99:197–209. [Google Scholar]
- 50.Niyogi K, Björkman O, Grossman A. Plant Cell. 1997;9:1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goss R, Bohme K, Wilhelm C. Planta. 1998;205:613–621. [Google Scholar]
- 52.Walters R G, Ruban A V, Horton P. Proc Natl Acad Sci USA. 1996;93:14204–14209. doi: 10.1073/pnas.93.24.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]