Abstract
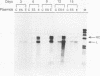
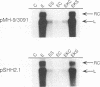
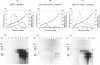
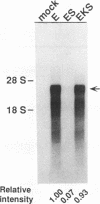
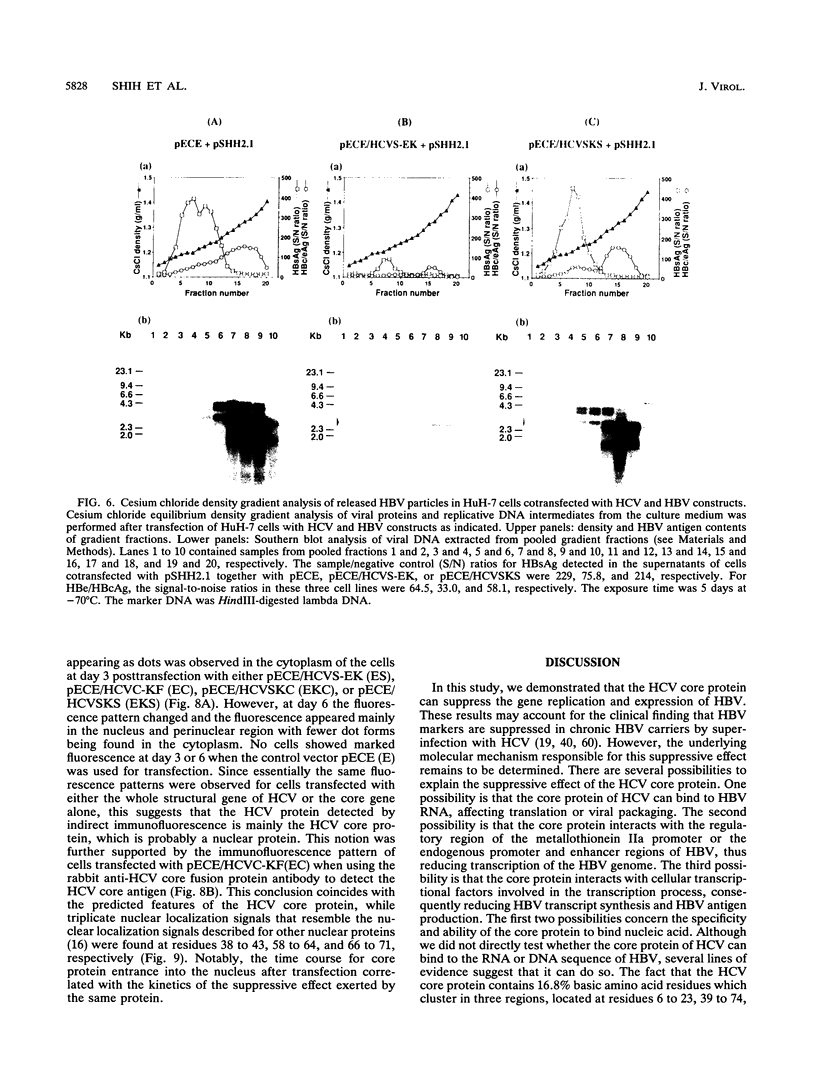
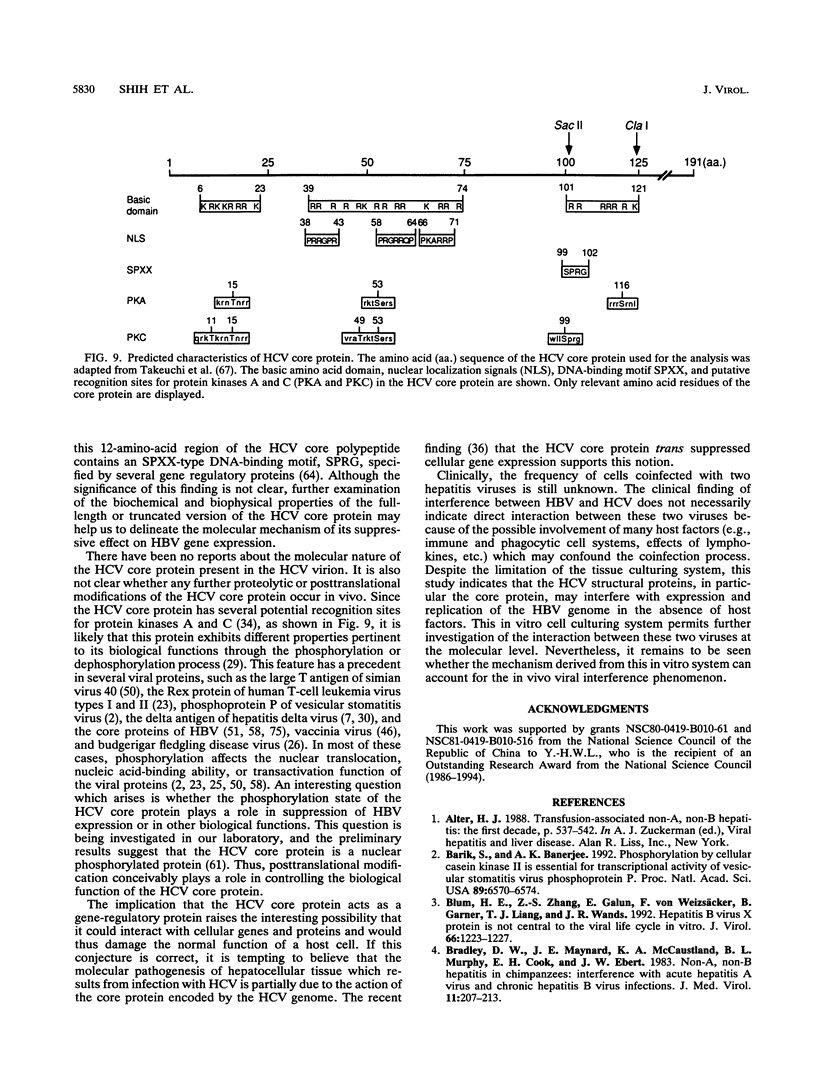
Hepatitis B and C viruses (HBV and HCV, respectively) are associated with acute and chronic liver diseases and hepatocellular carcinoma. To elucidate the molecular status of superinfection with these two hepatitis viruses, we cotransfected the full-length or truncated version of HCV structural genes (core and envelope 1) together with the cloned HBV DNA into a human hepatoma cell line (HuH-7). Expression of HBV-specific major transcripts (3.5 and 2.1 kb), as well as HBV antigens (hepatitis B surface antigen and hepatitis B e and core antigens), was reduced about two- to fourfold by the presence of the HCV structural genes. In addition, the secretion of HBV viral particles, including the viral nucleocapsid and mature virion, was drastically suppressed about 20-fold. Analysis of the intracellular HBV core protein-associated nucleic acid indicated that the encapsidated HBV pregenomic RNA was similarly reduced about 14-fold. Deletion analysis of the HCV structural genes demonstrated that the core gene alone or the fragment containing the core protein's N-terminal 122 amino acid residues conferred the same level of suppressive activity as the full-length structural genes. By indirect immunofluorescence, we found that the core protein of HCV was located in the cytoplasm of transfected HuH-7 cells at day 3 posttransfection and was targeted to the nucleus at day 6. Thus, the kinetics of the suppressive effect exerted by HCV constructs matched the timing of core protein entrance into the nucleus. Our results substantiate the clinical finding that HBV markers are suppressed by superinfection with HCV and further imply that this inhibitory effect may occur in the processes of transcription and encapsidation of HBV pregenomic RNA and may be mediated by the core protein of HCV. The deduced amino acid sequence of the HCV core protein has revealed that it is a basic protein which contains a putative DNA-binding motif (SPRG), as well as triplicate nuclear localization signals and several putative protein kinase A and C recognition sites. These characteristics imply that the HCV core protein can also function as a gene-regulatory protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barik S., Banerjee A. K. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H. E., Zhang Z. S., Galun E., von Weizsäcker F., Garner B., Liang T. J., Wands J. R. Hepatitis B virus X protein is not central to the viral life cycle in vitro. J Virol. 1992 Feb;66(2):1223–1227. doi: 10.1128/jvi.66.2.1223-1227.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. W., Maynard J. E., McCaustland K. A., Murphy B. L., Cook E. H., Ebert J. W. Non-A, non-B hepatitis in chimpanzees: interference with acute hepatitis A virus and chronic hepatitis B virus infections. J Med Virol. 1983;11(3):207–213. doi: 10.1002/jmv.1890110304. [DOI] [PubMed] [Google Scholar]
- Bruix J., Barrera J. M., Calvet X., Ercilla G., Costa J., Sanchez-Tapias J. M., Ventura M., Vall M., Bruguera M., Bru C. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989 Oct 28;2(8670):1004–1006. doi: 10.1016/s0140-6736(89)91015-5. [DOI] [PubMed] [Google Scholar]
- Chan C. Y., Lee S. D., Wu J. C., Hwang S. J., Wang Y. J., Huang Y. S., Lo K. J. Superinfection with hepatitis C virus in patients with symptomatic chronic hepatitis B. Scand J Infect Dis. 1991;23(4):421–424. doi: 10.3109/00365549109075089. [DOI] [PubMed] [Google Scholar]
- Chang M. F., Baker S. C., Soe L. H., Kamahora T., Keck J. G., Makino S., Govindarajan S., Lai M. M. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988 Jul;62(7):2403–2410. doi: 10.1128/jvi.62.7.2403-2410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. F., Sun C. Y., Chen C. J., Chang S. C. Functional motifs of delta antigen essential for RNA binding and replication of hepatitis delta virus. J Virol. 1993 May;67(5):2529–2536. doi: 10.1128/jvi.67.5.2529-2536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. S., Kuo G. C., Sung J. L., Lai M. Y., Sheu J. C., Chen P. J., Yang P. M., Hsu H. M., Chang M. H., Chen C. J. Hepatitis C virus infection in an area hyperendemic for hepatitis B and chronic liver disease: the Taiwan experience. J Infect Dis. 1990 Oct;162(4):817–822. doi: 10.1093/infdis/162.4.817. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Lin M. H., Tai K. F., Liu P. C., Lin C. J., Chen D. S. The Taiwanese hepatitis C virus genome: sequence determination and mapping the 5' termini of viral genomic and antigenomic RNA. Virology. 1992 May;188(1):102–113. doi: 10.1016/0042-6822(92)90739-c. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Kuo G., Choo Q. L., Donato M. F., Del Ninno E., Tommasini M. A., Dioguardi N., Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989 Oct 28;2(8670):1006–1008. doi: 10.1016/s0140-6736(89)91016-7. [DOI] [PubMed] [Google Scholar]
- Deppert W., Walter G. Domains of simian virus 40 large T-antigen exposed on the cell surface. Virology. 1982 Oct 15;122(1):56–70. doi: 10.1016/0042-6822(82)90377-4. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fattovich G., Tagger A., Brollo L., Giustina G., Pontisso P., Realdi G., Alberti A., Ruol A. Hepatitis C virus infection in chronic hepatitis B virus carriers. J Infect Dis. 1991 Feb;163(2):400–402. doi: 10.1093/infdis/163.2.400. [DOI] [PubMed] [Google Scholar]
- Fong T. L., Di Bisceglie A. M., Waggoner J. G., Banks S. M., Hoofnagle J. H. The significance of antibody to hepatitis C virus in patients with chronic hepatitis B. Hepatology. 1991 Jul;14(1):64–67. doi: 10.1002/hep.1840140111. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grakoui A., Wychowski C., Lin C., Feinstone S. M., Rice C. M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993 Mar;67(3):1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. L., Yip M. T., Xie Y., Chen I. S. Phosphorylation regulates RNA binding by the human T-cell leukemia virus Rex protein. J Virol. 1992 Jul;66(7):4325–4330. doi: 10.1128/jvi.66.7.4325-4330.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
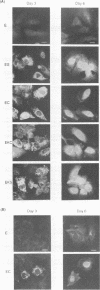
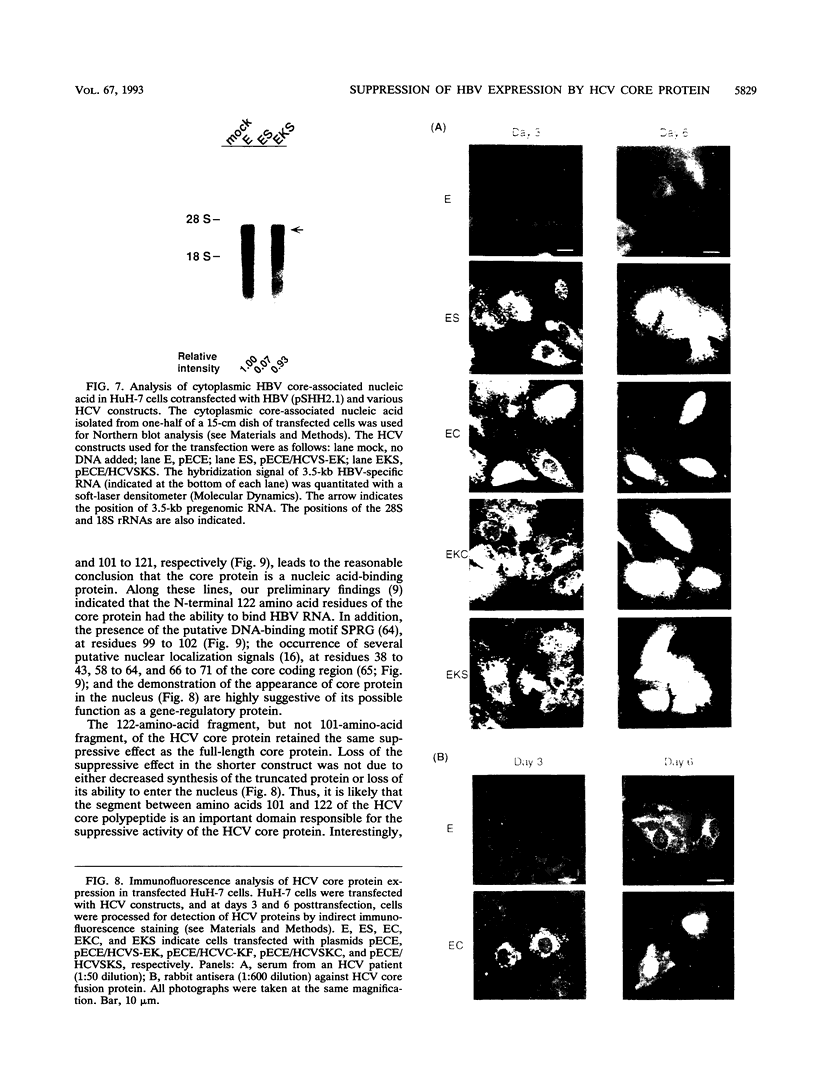
- Harada S., Watanabe Y., Takeuchi K., Suzuki T., Katayama T., Takebe Y., Saito I., Miyamura T. Expression of processed core protein of hepatitis C virus in mammalian cells. J Virol. 1991 Jun;65(6):3015–3021. doi: 10.1128/jvi.65.6.3015-3021.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton T., Zhou S., Standring D. N. RNA- and DNA-binding activities in hepatitis B virus capsid protein: a model for their roles in viral replication. J Virol. 1992 Sep;66(9):5232–5241. doi: 10.1128/jvi.66.9.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J. I., 2nd, Consigli R. A. Phosphorylation of the budgerigar fledgling disease virus major capsid protein VP1. J Virol. 1992 Jul;66(7):4551–4555. doi: 10.1128/jvi.66.7.4551-4555.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Kato N., Ootsuyama Y., Nakagawa M., Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska J. F., Robinson W. S. The proteins of hepatitis B Dane particle cores. J Med Virol. 1977;1(2):119–131. doi: 10.1002/jmv.1890010205. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Lee C. Z., Lai M. M. Hepatitis delta antigen expressed by recombinant baculoviruses: comparison of biochemical properties and post-translational modifications between the large and small forms. Virology. 1992 Sep;190(1):413–422. doi: 10.1016/0042-6822(92)91227-l. [DOI] [PubMed] [Google Scholar]
- Jeng K. S., Hu C. P., Chang C. M. Differential formation of disulfide linkages in the core antigen of extracellular and intracellular hepatitis B virus core particles. J Virol. 1991 Jul;65(7):3924–3927. doi: 10.1128/jvi.65.7.3924-3927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker M., Galle P., Schaller H. Expression and replication of the hepatitis B virus genome under foreign promoter control. Nucleic Acids Res. 1987 Dec 23;15(24):10117–10132. doi: 10.1093/nar/15.24.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kew M. C., Houghton M., Choo Q. L., Kuo G. Hepatitis C virus antibodies in southern African blacks with hepatocellular carcinoma. Lancet. 1990 Apr 14;335(8694):873–874. doi: 10.1016/0140-6736(90)90474-j. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavine J., Hirsch R., Ganem D. A system for studying the selective encapsidation of hepadnavirus RNA. J Virol. 1989 Oct;63(10):4257–4263. doi: 10.1128/jvi.63.10.4257-4263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. G., Lo S. J. Evidence for involvement of a ribosomal leaky scanning mechanism in the translation of the hepatitis B virus pol gene from the viral pregenome RNA. Virology. 1992 May;188(1):342–352. doi: 10.1016/0042-6822(92)90763-f. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Harada S., Suzuki R., Watanabe Y., Inoue Y., Saito I., Miyamura T. Expression of processed envelope protein of hepatitis C virus in mammalian and insect cells. J Virol. 1992 Mar;66(3):1425–1431. doi: 10.1128/jvi.66.3.1425-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatche N., Keller S. J. Phosphorylation of vaccinia virus core proteins during transcription in vitro. J Virol. 1991 May;65(5):2555–2561. doi: 10.1128/jvi.65.5.2555-2561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Okamoto H., Kurai K., Okada S., Yamamoto K., Lizuka H., Tanaka T., Fukuda S., Tsuda F., Mishiro S. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992 May;188(1):331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Kurai K., Iizuka H., Machida A., Miyakawa Y., Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991 Nov;72(Pt 11):2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- Rihs H. P., Jans D. A., Fan H., Peters R. The rate of nuclear cytoplasmic protein transport is determined by the casein kinase II site flanking the nuclear localization sequence of the SV40 T-antigen. EMBO J. 1991 Mar;10(3):633–639. doi: 10.1002/j.1460-2075.1991.tb07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J., Siddiqui A. In vivo phosphorylation and protein analysis of hepatitis B virus core antigen. J Virol. 1987 Apr;61(4):955–961. doi: 10.1128/jvi.61.4.955-961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury S., Faruqi A. F., Shih C. Pregenomic RNA encapsidation analysis of eleven missense and nonsense polymerase mutants of human hepatitis B virus. J Virol. 1991 Jul;65(7):3617–3624. doi: 10.1128/jvi.65.7.3617-3624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Miyamura T., Ohbayashi A., Harada H., Katayama T., Kikuchi S., Watanabe Y., Koi S., Onji M., Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbolli G., Zanetti A. R., Tanzi E., Cavanna L., Civardi G., Fornari F., Di Stasi M., Buscarini L. Serum antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. J Med Virol. 1990 Mar;30(3):230–232. doi: 10.1002/jmv.1890300316. [DOI] [PubMed] [Google Scholar]
- Schlicht H. J., Bartenschlager R., Schaller H. The duck hepatitis B virus core protein contains a highly phosphorylated C terminus that is essential for replication but not for RNA packaging. J Virol. 1989 Jul;63(7):2995–3000. doi: 10.1128/jvi.63.7.2995-3000.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells M. A., Chen M. L., Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen I. S., Liaw Y. F., Chu C. M., Pao C. C. Role of hepatitis C virus infection in spontaneous hepatitis B surface antigen clearance during chronic hepatitis B virus infection. J Infect Dis. 1992 May;165(5):831–834. doi: 10.1093/infdis/165.5.831. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Sánchez-Tapias J. M., Barrera J. M., Costa J., Ercilla M. G., Parés A., Comalrrena L., Soley F., Bruix J., Calvet X., Gil M. P. Hepatitis C virus infection in patients with nonalcoholic chronic liver disease. Ann Intern Med. 1990 Jun 15;112(12):921–924. doi: 10.7326/0003-4819-112-12-921. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. Nucleotide sequence of core and envelope genes of the hepatitis C virus genome derived directly from human healthy carriers. Nucleic Acids Res. 1990 Aug 11;18(15):4626–4626. doi: 10.1093/nar/18.15.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. The putative nucleocapsid and envelope protein genes of hepatitis C virus determined by comparison of the nucleotide sequences of two isolates derived from an experimentally infected chimpanzee and healthy human carriers. J Gen Virol. 1990 Dec;71(Pt 12):3027–3033. doi: 10.1099/0022-1317-71-12-3027. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiquaye K. N., Portmann B., Tovey G., Kessler H., Hu S., Lu X. Z., Zuckerman A. J., Craske J., Williams R. Non-A, non-B hepatitis in persistent carriers of hepatitis B virus. J Med Virol. 1983;11(3):179–189. doi: 10.1002/jmv.1890110302. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Cattaneo R., Koch H. G., Darai G., Schaller H., Schellekens H., van Eerd P. M., Deinhardt F. Cloned HBV DNA causes hepatitis in chimpanzees. Nature. 1982 Oct 21;299(5885):740–742. doi: 10.1038/299740a0. [DOI] [PubMed] [Google Scholar]
- Yaginuma K., Shirakata Y., Kobayashi M., Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci U S A. 1987 May;84(9):2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. T., Ou J. H. Phosphorylation of hepatitis B virus precore and core proteins. J Virol. 1991 May;65(5):2327–2331. doi: 10.1128/jvi.65.5.2327-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]