Abstract
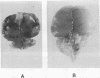
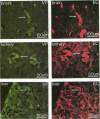
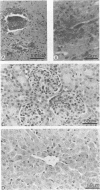
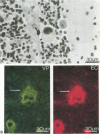
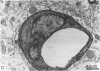
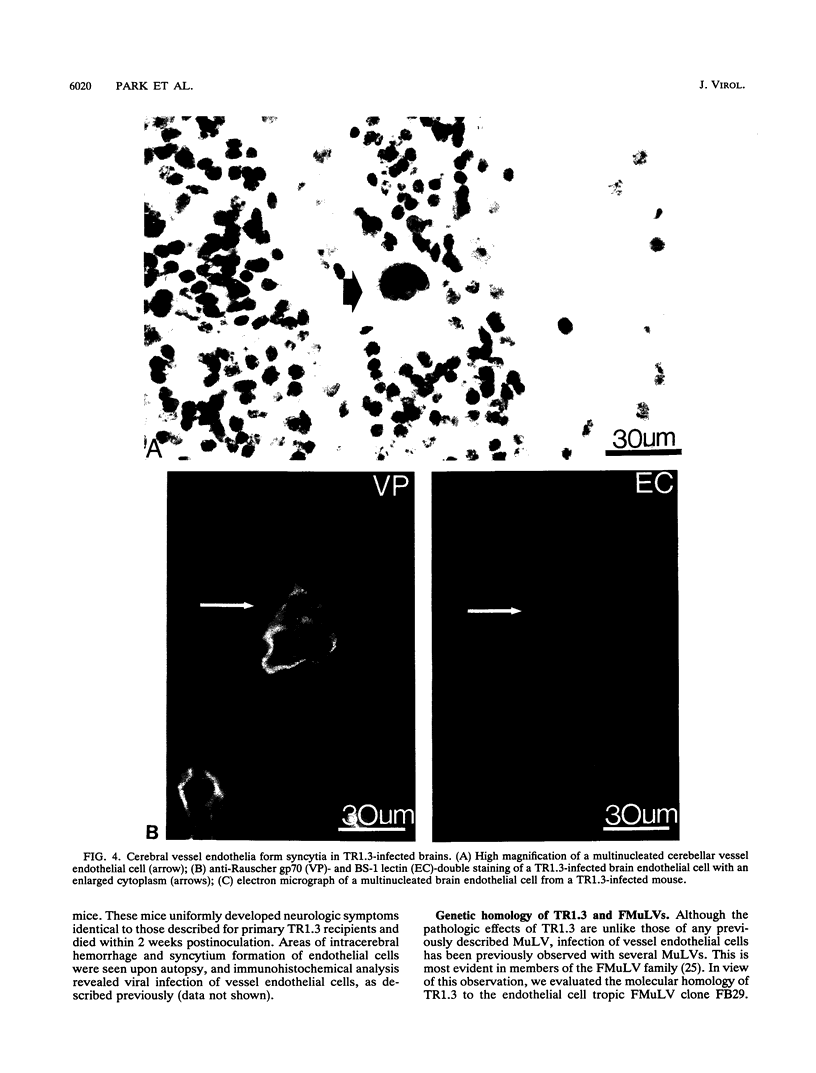
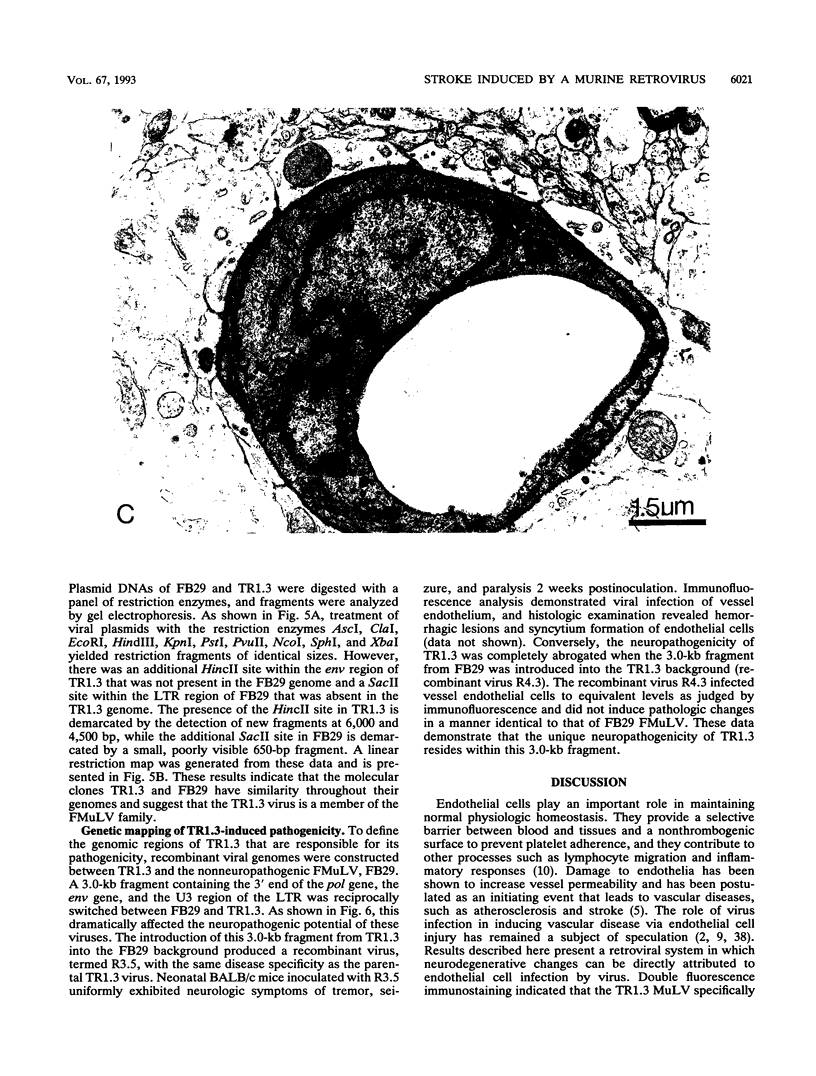
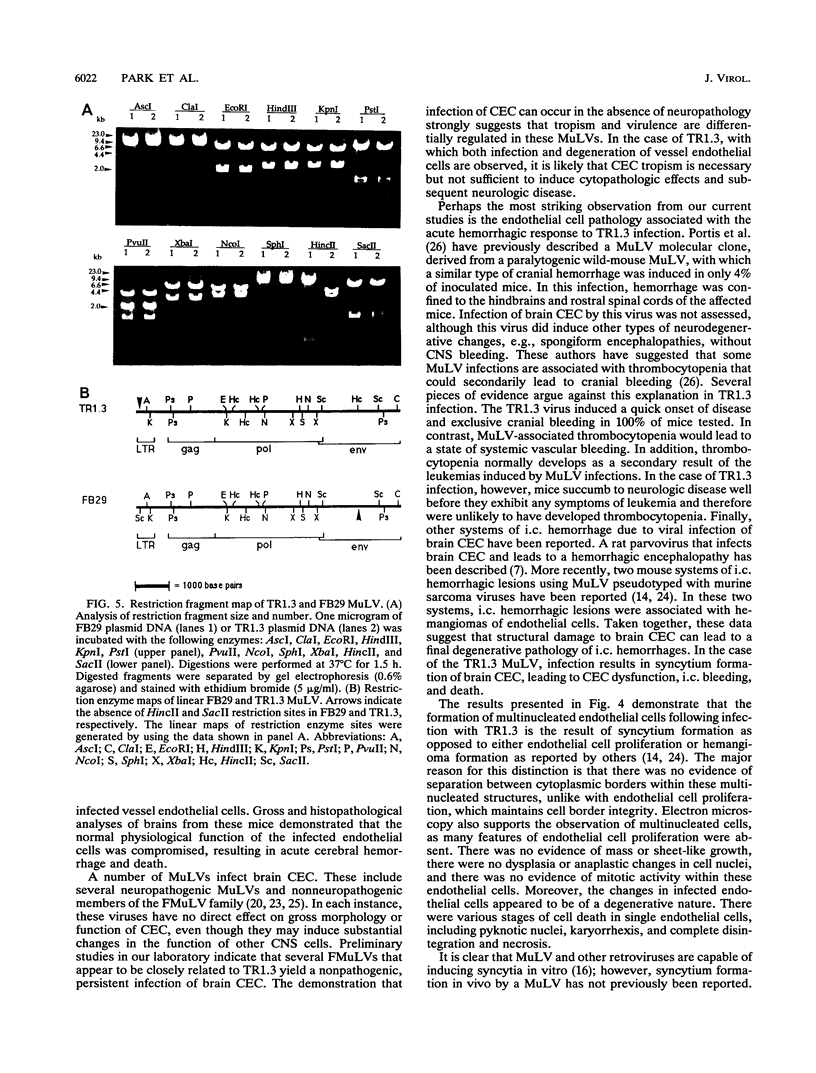
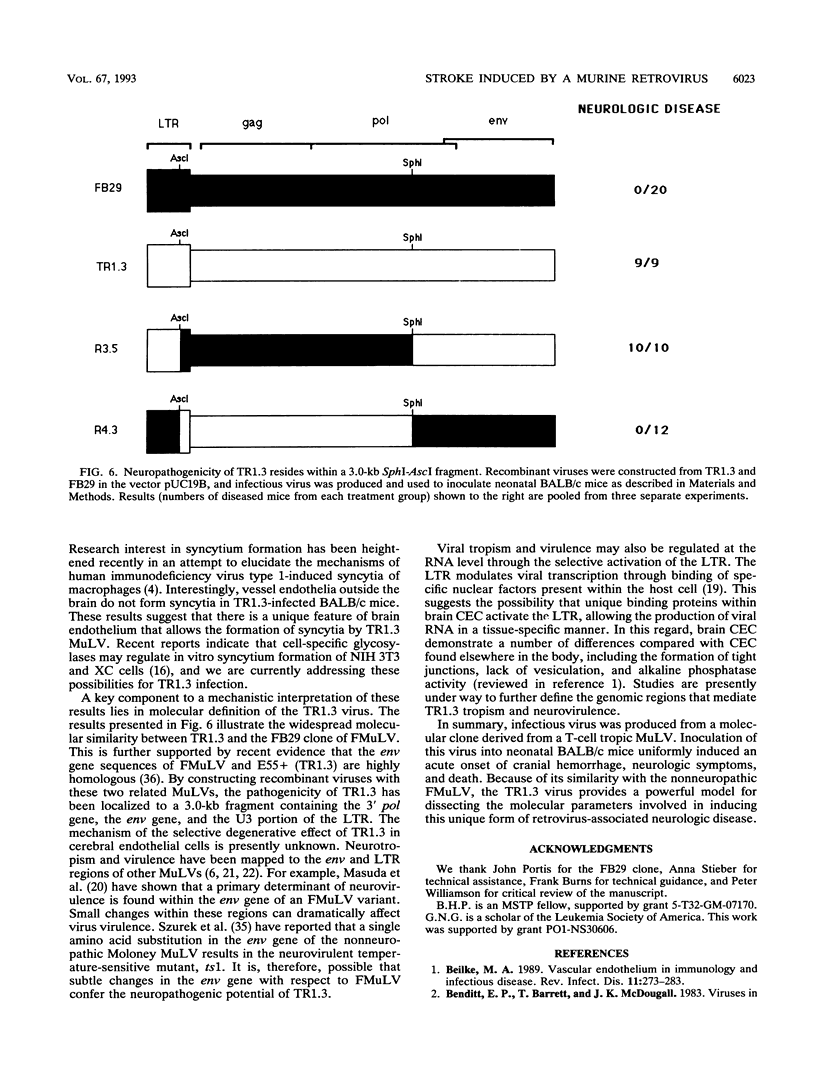
The mechanisms of endothelial cell damage that lead to cerebral hemorrhage are not completely understood. In this study, a cloned murine retrovirus, TR1.3, that uniformly induced stroke in neonatal BALB/c mice is described. Restriction digest mapping suggests that TR1.3 is part of the Friend murine leukemia virus (FMuLV) family. However, unlike mice exposed to other FMuLVs, mice infected with TR1.3 virus developed tremors and seizures within 8 to 18 days postinoculation. This was uniformly followed by paralysis and death within 1 to 2 days. Postmortem examination of TR1.3-inoculated mice revealed edematous brain tissue with large areas of intracerebral hemorrhage. Histologic analysis revealed prominent small vessel pathology including syncytium formation of endothelial cells. Immunohistochemical analysis of frozen brain sections using double fluorescence staining demonstrated that TR1.3 virus specifically infected small vessel endothelial cells. Although infection of vessel endothelial cells was detected in several organs, only brain endothelial cells displayed viral infection associated with hemorrhage. The primary determinant of TR1.3-induced neuropathogenicity was found to reside within a 3.0-kb fragment containing the 3' end of the pol gene, the env gene, and the U3 region of the long terminal repeat. The restricted tropism and acute pathogenicity of this cloned murine retrovirus provide a model for studying virus-induced stroke and for elucidating the mechanisms involved in syncytium formation by retroviruses in vivo.
Full text
PDF

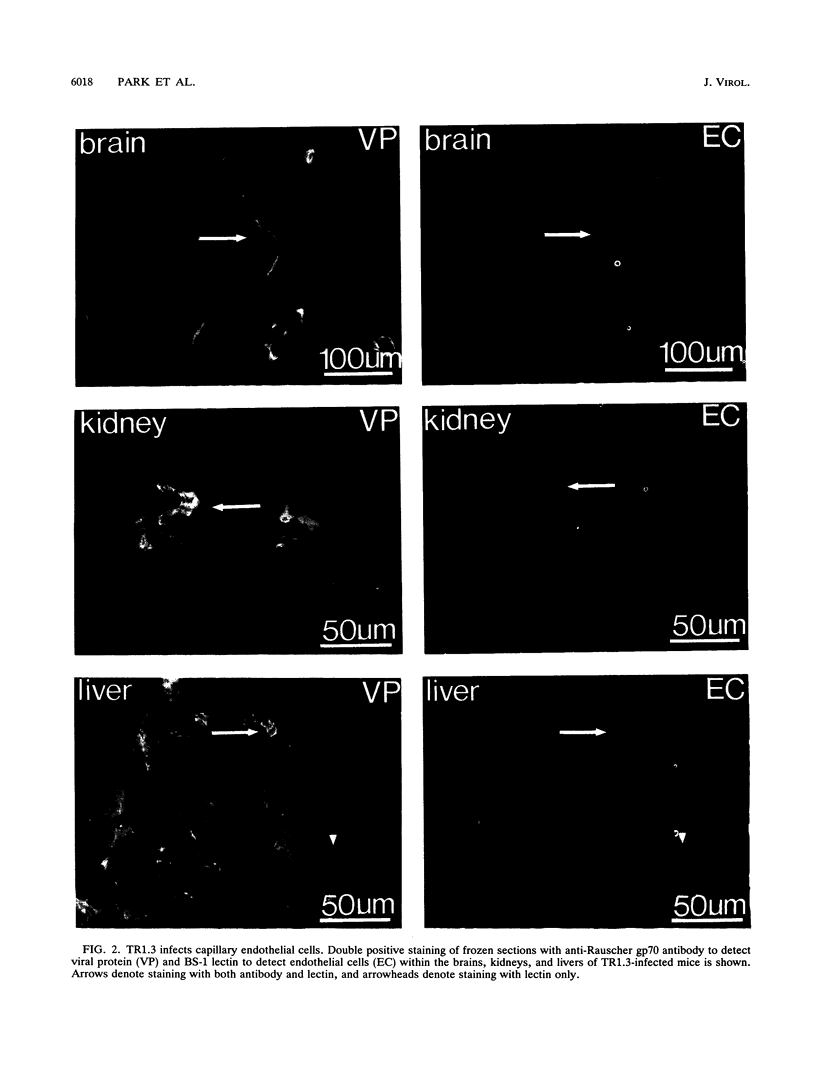


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beilke M. A. Vascular endothelium in immunology and infectious disease. Rev Infect Dis. 1989 Mar-Apr;11(2):273–283. doi: 10.1093/clinids/11.2.273. [DOI] [PubMed] [Google Scholar]
- Carneiro A. V., Ferro J., Figueiredo C., Costa L., Campos J., de Pádua F. Herpes zoster and controlateral hemiplegia in an African patient infected with HIV-1. Acta Med Port. 1991 Mar-Apr;4(2):91–92. [PubMed] [Google Scholar]
- Chowdhury M. I., Koyanagi Y., Suzuki M., Kobayashi S., Yamaguchi K., Yamamoto N. Increased production of human immunodeficiency virus (HIV) in HIV-induced syncytia formation: an efficient infection process. Virus Genes. 1992 Jan;6(1):63–78. doi: 10.1007/BF01703758. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Robitaille Y., Jolicoeur P. Retrovirus-induced spongiform encephalopathy: the 3'-end long terminal repeat-containing viral sequences influence the incidence of the disease and the specificity of the neurological syndrome. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8818–8822. doi: 10.1073/pnas.82.24.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldadah A. H., Nathanson N., Smith K. O., Squire R. A., Santos G. W., Melby E. C. Viral hemorrhagic encephalopathy of rats. Science. 1967 Apr 21;156(3773):392–394. doi: 10.1126/science.156.3773.392. [DOI] [PubMed] [Google Scholar]
- Engstrom J. W., Lowenstein D. H., Bredesen D. E. Cerebral infarctions and transient neurologic deficits associated with acquired immunodeficiency syndrome. Am J Med. 1989 May;86(5):528–532. doi: 10.1016/0002-9343(89)90379-3. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G. Atherosclerosis: the consequence of infection with a herpesvirus. Adv Vet Sci Comp Med. 1985;30:39–66. [PubMed] [Google Scholar]
- Friedman H. M., Macarak E. J., MacGregor R. R., Wolfe J., Kefalides N. A. Virus infection of endothelial cells. J Infect Dis. 1981 Feb;143(2):266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Gardner M. B. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis. 1985 Jan-Feb;7(1):99–110. doi: 10.1093/clinids/7.1.99. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Blank K. J. T cell receptor genes and T cell development in virus-transformed early T cell lines. J Immunol. 1990 Feb 15;144(4):1518–1525. [PubMed] [Google Scholar]
- Hevezi P., Goff S. P. Generation of recombinant murine retroviral genomes containing the v-src oncogene: isolation of a virus inducing hemangiosarcomas in the brain. J Virol. 1991 Oct;65(10):5333–5341. doi: 10.1128/jvi.65.10.5333-5341.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones J. S., Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993 Jan;67(1):67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J. 1987 Apr;19(4):225–234. doi: 10.1007/BF01680633. [DOI] [PubMed] [Google Scholar]
- Lynch W. P., Czub S., McAtee F. J., Hayes S. F., Portis J. L. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron. 1991 Sep;7(3):365–379. doi: 10.1016/0896-6273(91)90289-c. [DOI] [PubMed] [Google Scholar]
- Manley N. R., O'Connell M. A., Sharp P. A., Hopkins N. Nuclear factors that bind to the enhancer region of nondefective Friend murine leukemia virus. J Virol. 1989 Oct;63(10):4210–4223. doi: 10.1128/jvi.63.10.4210-4223.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M., Remington M. P., Hoffman P. M., Ruscetti S. K. Molecular characterization of a neuropathogenic and nonerythroleukemogenic variant of Friend murine leukemia virus PVC-211. J Virol. 1992 May;66(5):2798–2806. doi: 10.1128/jvi.66.5.2798-2806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Kay D. G., Rassart E., Robitaille Y., Jolicoeur P. Substitution of the U3 long terminal repeat region of the neurotropic Cas-Br-E retrovirus affects its disease-inducing potential. J Virol. 1990 Aug;64(8):3742–3752. doi: 10.1128/jvi.64.8.3742-3752.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts O. M., Powers J. M., Bilello J. A., Hoffman P. M. Ultrastructural changes associated with retroviral replication in central nervous system capillary endothelial cells. Lab Invest. 1987 Apr;56(4):401–409. [PubMed] [Google Scholar]
- Pitts O. M., Powers J. M., Hoffman P. M. Vascular neoplasms induced in rodent central nervous system by murine sarcoma viruses. Lab Invest. 1983 Aug;49(2):171–182. [PubMed] [Google Scholar]
- Portis J. L., Czub S., Garon C. F., McAtee F. J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990 Apr;64(4):1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L. Wild mouse retrovirus: pathogenesis. Curr Top Microbiol Immunol. 1990;160:11–27. doi: 10.1007/978-3-642-75267-4_2. [DOI] [PubMed] [Google Scholar]
- Rawlinson W. D., Cunningham A. L. Contralateral hemiplegia following thoracic herpes zoster. Med J Aust. 1991 Sep 2;155(5):344–346. doi: 10.5694/j.1326-5377.1991.tb142297.x. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scaravilli F., Daniel S. E., Harcourt-Webster N., Guiloff R. J. Chronic basal meningitis and vasculitis in acquired immunodeficiency syndrome. A possible role for human immunodeficiency virus. Arch Pathol Lab Med. 1989 Feb;113(2):192–195. [PubMed] [Google Scholar]
- Sharpe A. H., Hunter J. J., Chassler P., Jaenisch R. Role of abortive retroviral infection of neurons in spongiform CNS degeneration. Nature. 1990 Jul 12;346(6280):181–183. doi: 10.1038/346181a0. [DOI] [PubMed] [Google Scholar]
- Shuper A., Vining E. P., Freeman J. M. Central nervous system vasculitis after chickenpox--cause or coincidence? Arch Dis Child. 1990 Nov;65(11):1245–1248. doi: 10.1136/adc.65.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonian N. A., Rosenthal L. A., Korostoff J., Hickey W. F., Blank K. J., Gaulton G. N. Specific infection of central nervous system white matter by a variant of gross murine leukemia virus. Virology. 1990 Jul;177(1):384–387. doi: 10.1016/0042-6822(90)90496-e. [DOI] [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Ball J. K., Wong P. K. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990 Feb;64(2):467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumas K. M., Poszgay J. M., Avidan N., Ksiazek S. J., Overmoyer B., Blank K. J., Prystowsky M. B. Loss of antigenic epitopes as the result of env gene recombination in retrovirus-induced leukemia in immunocompetent mice. Virology. 1993 Feb;192(2):587–595. doi: 10.1006/viro.1993.1075. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]