Abstract
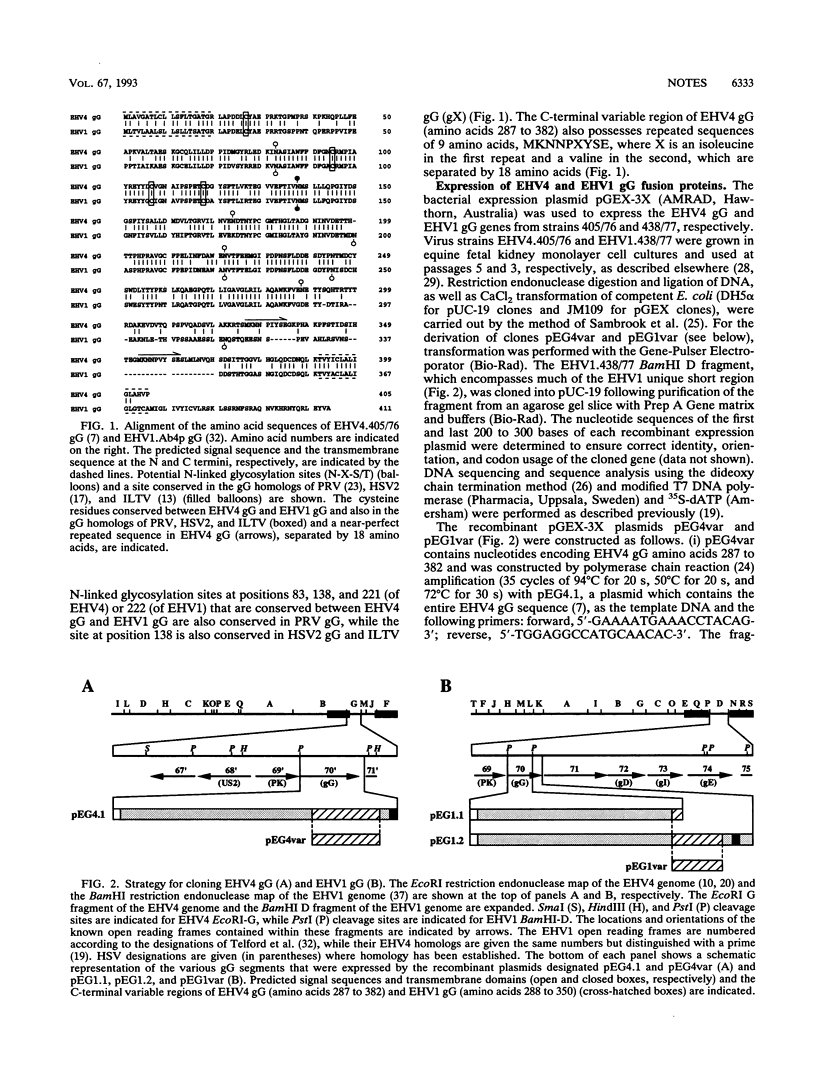
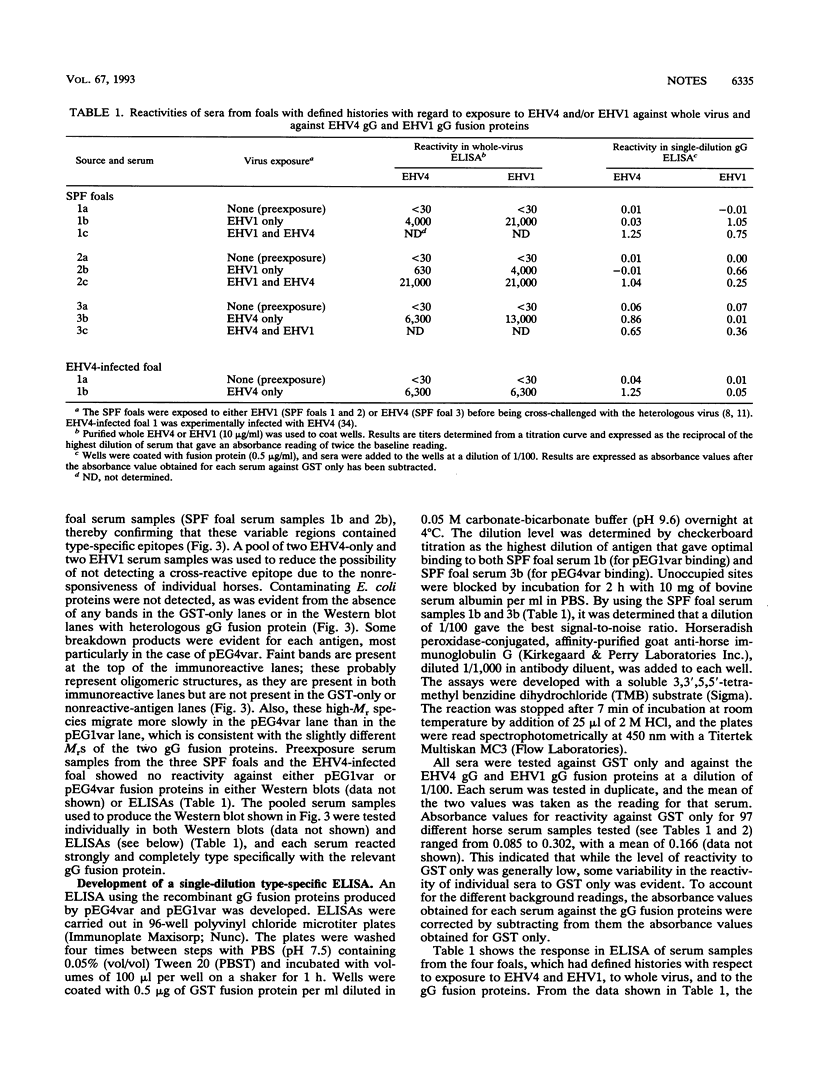
Specific serological diagnosis of equine herpesvirus 4 (EHV4; equine rhinopneumonitis virus) and EHV1 (equine abortion virus) hitherto has not been possible because of extensive antigenic cross-reactivity between these two closely related but distinct viruses. Recently, we identified EHV4 glycoprotein G (gG) and characterized it as a type-specific, secreted glycoprotein (B. S. Crabb, H. S. Nagesha, and M. J. Studdert, Virology 190:143-154, 1992). This paper shows that EHV1 gG also possesses type-specific epitopes and describes the localization of strong, type-specific epitopes to the apparently corresponding and highly variable regions comprising amino acids 287 to 382 of EHV4 gG and 288 to 350 of EHV1 gG. Fusion proteins expressing these variable regions reacted strongly and type specifically with sera from four foals, three of which were colostrum-deprived, specific-pathogen-free foals, whose history with respect to exposure to EHV4 or EHV1 was well-defined. These antigens provided the basis for the development of a single-well diagnostic enzyme-linked immunosorbent assay to distinguish horses infected with EHV4, EHV1, or both. Such a type-specific test provides for the first time the opportunity to differentiate antibodies to these viruses, and it has, therefore, important implications for understanding the epidemiology of these equine pathogens. Evidence for the existence of EHV1 in Australia 10 years prior to the first confirmed case of EHV1 abortion is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Bryans J. T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- Allen G. P., Coogle L. D. Characterization of an equine herpesvirus type 1 gene encoding a glycoprotein (gp13) with homology to herpes simplex virus glycoprotein C. J Virol. 1988 Aug;62(8):2850–2858. doi: 10.1128/jvi.62.8.2850-2858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., Yeargan M. R. Use of lambda gt11 and monoclonal antibodies to map the genes for the six major glycoproteins of equine herpesvirus 1. J Virol. 1987 Aug;61(8):2454–2461. doi: 10.1128/jvi.61.8.2454-2461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb B. S., Allen G. P., Studdert M. J. Characterization of the major glycoproteins of equine herpesviruses 4 and 1 and asinine herpesvirus 3 using monoclonal antibodies. J Gen Virol. 1991 Sep;72(Pt 9):2075–2082. doi: 10.1099/0022-1317-72-9-2075. [DOI] [PubMed] [Google Scholar]
- Crabb B. S., Nagesha H. S., Studdert M. J. Identification of equine herpesvirus 4 glycoprotein G: a type-specific, secreted glycoprotein. Virology. 1992 Sep;190(1):143–154. doi: 10.1016/0042-6822(92)91200-e. [DOI] [PubMed] [Google Scholar]
- Crabb B. S., Studdert M. J. Comparative studies of the proteins of equine herpesviruses 4 and 1 and asinine herpesvirus 3: antibody response of the natural hosts. J Gen Virol. 1990 Sep;71(Pt 9):2033–2041. doi: 10.1099/0022-1317-71-9-2033. [DOI] [PubMed] [Google Scholar]
- Cullinane A. A., Rixon F. J., Davison A. J. Characterization of the genome of equine herpesvirus 1 subtype 2. J Gen Virol. 1988 Jul;69(Pt 7):1575–1590. doi: 10.1099/0022-1317-69-7-1575. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Studdert M. J. Immunologic relationships between equine herpesvirus type 1 (equine abortion virus) and type 4 (equine rhinopneumonitis virus). Am J Vet Res. 1984 Oct;45(10):1947–1952. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács F., Mettenleiter T. C. Firefly luciferase as a marker for herpesvirus (pseudorabies virus) replication in vitro and in vivo. J Gen Virol. 1991 Dec;72(Pt 12):2999–3008. doi: 10.1099/0022-1317-72-12-2999. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. Evolutionary relationships of virion glycoprotein genes in the S regions of alphaherpesvirus genomes. J Gen Virol. 1990 Oct;71(Pt 10):2361–2367. doi: 10.1099/0022-1317-71-10-2361. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Moss H. W., McNab D., Frame M. C. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J Gen Virol. 1987 Jan;68(Pt 1):19–38. doi: 10.1099/0022-1317-68-1-19. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Rauh I. A glycoprotein gX-beta-galactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990 Oct;30(1):55–65. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., Crabb B. S., Studdert M. J. Analysis of the nucleotide sequence of five genes at the left end of the unique short region of the equine herpesvirus 4 genome. Arch Virol. 1993;128(1-2):143–154. doi: 10.1007/BF01309795. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., McNeil J. R., Ficorilli N., Studdert M. J. Cloning and restriction endonuclease mapping of the genome of an equine herpesvirus 4 (equine rhinopneumonitis virus), strain 405/76. Arch Virol. 1992;124(3-4):379–387. doi: 10.1007/BF01309818. [DOI] [PubMed] [Google Scholar]
- Nicolson L., Onions D. E. The nucleotide sequence of the equine herpesvirus 4 gC gene homologue. Virology. 1990 Nov;179(1):378–387. doi: 10.1016/0042-6822(90)90305-b. [DOI] [PubMed] [Google Scholar]
- Post L. E., Thomsen D. R., Petrovskis E. A., Meyer A. L., Berlinski P. J., Wardley R. C. Genetic engineering of the pseudorabies virus genome to construct live vaccines. J Reprod Fertil Suppl. 1990;41:97–104. [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Blackney M. H. Equine herpesviruses: on the differentiation of respiratory from foetal strains of type 1. Aust Vet J. 1979 Oct;55(10):488–492. doi: 10.1111/j.1751-0813.1979.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Crabb B. S., Ficorilli N. The molecular epidemiology of equine herpesvirus 1 (equine abortion virus) in Australasia 1975 to 1989. Aust Vet J. 1992 May;69(5):104–111. doi: 10.1111/j.1751-0813.1992.tb07462.x. [DOI] [PubMed] [Google Scholar]
- Studdert M. J. Restriction endonuclease DNA fingerprinting of respiratory, foetal and perinatal foal isolates of equine herpesvirus type 1. Arch Virol. 1983;77(2-4):249–258. doi: 10.1007/BF01309272. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Simpson T., Roizman B. Differentiation of respiratory and abortigenic isolates of equine herpesvirus 1 by restriction endonucleases. Science. 1981 Oct 30;214(4520):562–564. doi: 10.1126/science.6270790. [DOI] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Marchioli C. C., Yancey R. J., Jr, Post L. E. Replication and virulence of pseudorabies virus mutants lacking glycoprotein gX. J Virol. 1987 Jan;61(1):229–232. doi: 10.1128/jvi.61.1.229-232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J., Studdert M. J., Peterson J. E. Equine herpes viruses. 2. Persistence of equine herpesviruses in experimentally infected horses and the experimental induction of abortion. Aust Vet J. 1970 Mar;46(3):90–98. doi: 10.1111/j.1751-0813.1970.tb15928.x. [DOI] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Davison A. J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981 Dec;57(Pt 2):307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]
- Whittaker G. R., Riggio M. P., Halliburton I. W., Killington R. A., Allen G. P., Meredith D. M. Antigenic and protein sequence homology between VP13/14, a herpes simplex virus type 1 tegument protein, and gp10, a glycoprotein of equine herpesvirus 1 and 4. J Virol. 1991 May;65(5):2320–2326. doi: 10.1128/jvi.65.5.2320-2326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker G. R., Taylor L. A., Elton D. M., Giles L. E., Bonass W. A., Halliburton I. W., Killington R. A., Meredith D. M. Glycoprotein 60 of equine herpesvirus type 1 is a homologue of herpes simplex virus glycoprotein D and plays a major role in penetration of cells. J Gen Virol. 1992 Apr;73(Pt 4):801–809. doi: 10.1099/0022-1317-73-4-801. [DOI] [PubMed] [Google Scholar]
- Whittaker G. R., Wheldon L. A., Giles L. E., Stocks J. M., Halliburton I. W., Killington R. A., Meredith D. M. Characterization of the high Mr glycoprotein (gP300) of equine herpesvirus type 1 as a novel glycoprotein with extensive O-linked carbohydrate. J Gen Virol. 1990 Oct;71(Pt 10):2407–2416. doi: 10.1099/0022-1317-71-10-2407. [DOI] [PubMed] [Google Scholar]
- Yeargan M. R., Allen G. P., Bryans J. T. Rapid subtyping of equine herpesvirus 1 with monoclonal antibodies. J Clin Microbiol. 1985 May;21(5):694–697. doi: 10.1128/jcm.21.5.694-697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T., Gielkens A. L., Moormann R. J., Berns A. J. Marker vaccines, virus protein-specific antibody assays and the control of Aujeszky's disease. Vet Microbiol. 1990 Jun;23(1-4):85–101. doi: 10.1016/0378-1135(90)90139-m. [DOI] [PubMed] [Google Scholar]
- van Zijl M., Wensvoort G., de Kluyver E., Hulst M., van der Gulden H., Gielkens A., Berns A., Moormann R. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol. 1991 May;65(5):2761–2765. doi: 10.1128/jvi.65.5.2761-2765.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



