Abstract
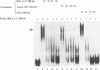
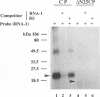
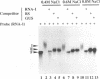
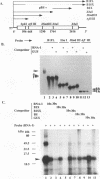
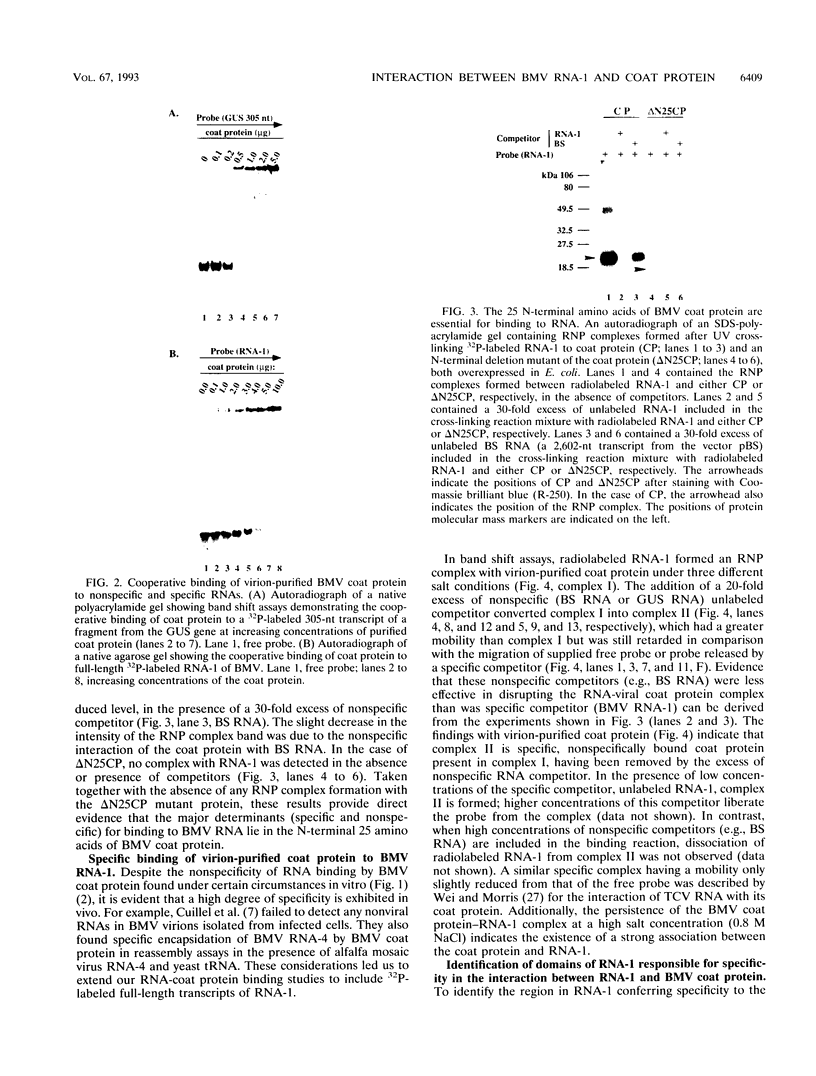
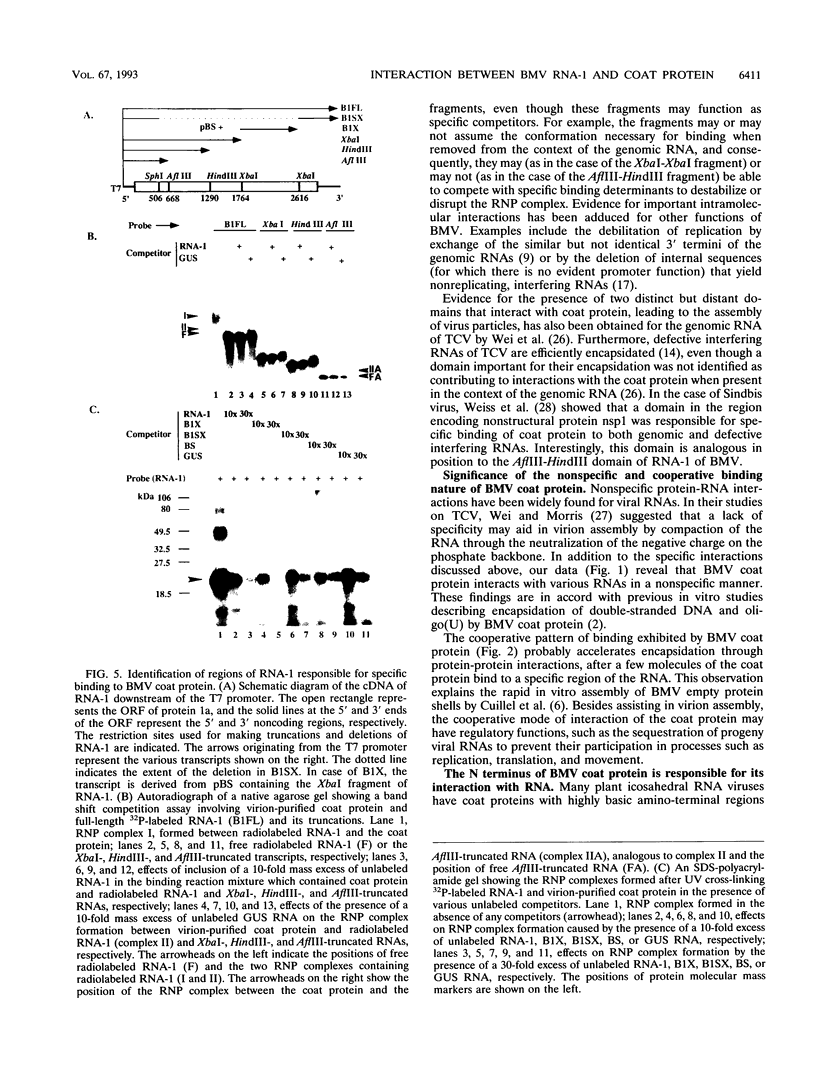
Even though many single-stranded RNAs are present in the cytoplasm of infected cells, encapsidation by brome mosaic virus (BMV) coat protein is specific for BMV RNA. Although the highly conserved 3' region of each of the three BMV genomic RNAs is an attractive candidate for the site of recognition by the coat protein, band shift and UV cross-linking assays in the presence of specific and nonspecific competitors revealed only nonspecific interactions. However, BMV RNA-1 formed a retarded complex (complex I) with the coat protein in the absence of competitors, and two domains of RNA-1 that specifically bound coat protein in a small complex (complex II), presumably early in the encapsidation process, were identified. Strong nonspecific, cooperative binding was observed in the presence of high concentrations of coat protein, suggesting that this provides the mechanism leading to rapid encapsidation seen in vivo. In contrast, no binding to a coat protein mutant lacking the N-terminal 25 amino acids that has been shown to be incapable of encapsidation in vivo (R. Sacher and P. Ahlquist, J. Virol. 63:4545-4552, 1989) was detected in vitro. The use of deletion mutants of RNA-1 revealed the presence of domains within the coding region of protein 1a that formed complexes with purified coat protein. One deletion mutant (B1SX) lacking these domains was only slightly more effective in dissociating RNA-1-coat protein complexes than were nonspecific competitors, further suggesting that regions other than the 3' end can participate in the selective encapsidation of BMV RNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Nucleotide sequence of the brome mosaic virus genome and its implications for viral replication. J Mol Biol. 1984 Feb 5;172(4):369–383. doi: 10.1016/s0022-2836(84)80012-1. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B., Hiebert E., Bracker C. E. The effects of various polyanions on shell formation of some spherical viruses. Virology. 1969 Dec;39(4):924–930. doi: 10.1016/0042-6822(69)90029-4. [DOI] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D. Cap-independent enhancement of translation by a plant potyvirus 5' nontranslated region. J Virol. 1990 Apr;64(4):1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Knorr D., Schuster G., Zambryski P. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990 Feb 23;60(4):637–647. doi: 10.1016/0092-8674(90)90667-4. [DOI] [PubMed] [Google Scholar]
- Cuillel M., Berthet-Colominas C., Krop B., Tardieu A., Vachette P., Jacrot B. Self-assembly of brome mosaic virus capsids. Kinetic study using neutron and X-ray solution scattering. J Mol Biol. 1983 Mar 15;164(4):645–650. doi: 10.1016/0022-2836(83)90055-4. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Rao A. L., Hall T. C. Replication in vivo of mutant brome mosaic virus RNAs defective in aminoacylation. J Mol Biol. 1989 Apr 5;206(3):425–438. doi: 10.1016/0022-2836(89)90491-9. [DOI] [PubMed] [Google Scholar]
- Duggal R., Rao A. L., Hall T. C. Unique nucleotide differences in the conserved 3' termini of brome mosaic virus RNAs are maintained through their optimization of genome replication. Virology. 1992 Mar;187(1):261–270. doi: 10.1016/0042-6822(92)90314-f. [DOI] [PubMed] [Google Scholar]
- Geigenmüller-Gnirke U., Nitschko H., Schlesinger S. Deletion analysis of the capsid protein of Sindbis virus: identification of the RNA binding region. J Virol. 1993 Mar;67(3):1620–1626. doi: 10.1128/jvi.67.3.1620-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. F., German T. L., Loesch-Fries L. S., Hall T. C. Highly active template-specific RNA-dependent RNA polymerase from barley leaves infected with brome mosaic virus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4956–4960. doi: 10.1073/pnas.76.10.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton L. A., Carrington J. C., Morris T. J. Turnip crinkle virus infection from RNA synthesized in vitro. Virology. 1989 May;170(1):214–218. doi: 10.1016/0042-6822(89)90368-1. [DOI] [PubMed] [Google Scholar]
- Lane L. C. The bromoviruses. Adv Virus Res. 1974;19:151–220. doi: 10.1016/s0065-3527(08)60660-0. [DOI] [PubMed] [Google Scholar]
- Marsh L. E., Huntley C. C., Pogue G. P., Connell J. P., Hall T. C. Regulation of (+):(-)-strand asymmetry in replication of brome mosaic virus RNA. Virology. 1991 May;182(1):76–83. doi: 10.1016/0042-6822(91)90650-z. [DOI] [PubMed] [Google Scholar]
- Marsh L. E., Pogue G. P., Szybiak U., Connell J. P., Hall T. C. Non-replicating deletion mutants of brome mosaic virus RNA-2 interfere with viral replication. J Gen Virol. 1991 Oct;72(Pt 10):2367–2374. doi: 10.1099/0022-1317-72-10-2367. [DOI] [PubMed] [Google Scholar]
- Nakhasi H. L., Rouault T. A., Haile D. J., Liu T. Y., Klausner R. D. Specific high-affinity binding of host cell proteins to the 3' region of rubella virus RNA. New Biol. 1990 Mar;2(3):255–264. [PubMed] [Google Scholar]
- Sacher R., Ahlquist P. Effects of deletions in the N-terminal basic arm of brome mosaic virus coat protein on RNA packaging and systemic infection. J Virol. 1989 Nov;63(11):4545–4552. doi: 10.1128/jvi.63.11.4545-4552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgro J. Y., Jacrot B., Chroboczek J. Identification of regions of brome mosaic virus coat protein chemically cross-linked in situ to viral RNA. Eur J Biochem. 1986 Jan 2;154(1):69–76. doi: 10.1111/j.1432-1033.1986.tb09360.x. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Stockley P. G., Harrison S. C. Structure and assembly of turnip crinkle virus. II. Mechanism of reassembly in vitro. J Mol Biol. 1986 Oct 20;191(4):639–658. doi: 10.1016/0022-2836(86)90451-1. [DOI] [PubMed] [Google Scholar]
- Wei N., Heaton L. A., Morris T. J., Harrison S. C. Structure and assembly of turnip crinkle virus. VI. Identification of coat protein binding sites on the RNA. J Mol Biol. 1990 Jul 5;214(1):85–95. doi: 10.1016/0022-2836(90)90148-F. [DOI] [PubMed] [Google Scholar]
- Wei N., Morris T. J. Interactions between viral coat protein and a specific binding region on turnip crinkle virus RNA. J Mol Biol. 1991 Dec 5;222(3):437–443. doi: 10.1016/0022-2836(91)90483-m. [DOI] [PubMed] [Google Scholar]
- Weiss B., Nitschko H., Ghattas I., Wright R., Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J Virol. 1989 Dec;63(12):5310–5318. doi: 10.1128/jvi.63.12.5310-5318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]