Abstract
The Cas-Br-E murine leukemia virus (MuLV) induces a progressive hindlimb paralysis accompanied by a spongiform myeloencephalopathy in susceptible mice. In order to better understand the pathological process leading to these neurodegenerative lesions, we have investigated the nature of the cell type(s) infected by the virus during the course of the disease in CFW/D and SWR/J mice. For this purpose, we used in situ hybridization with virus-specific probes in combination with cell-type-specific histochemical (lectin) and immunological markers as well as morphological assessment. In the early stage of infection, endothelial cells represented the main cell type expressing viral RNA in the central nervous system (CNS). With disease progression and the appearance of lesions, microglial cells became the major cell type infected, accounting for up to 65% of the total infected cell population in diseased areas. Morphologically, these cells appeared activated and were frequently found in clusters. Infection and activation of microglial cells were almost exclusively restricted to diseased regions of the CNS. Neurons in diseased regions were not discernibly infected with virus at either early or late times of disease progression. Similarly, the proportion of infected astrocytes was typically < 1%. Although some endothelial cells and oligodendrocytes were infected by the virus, their infection was not limited to diseased CNS regions. These results are consistent with a model of indirect motor neuron degeneration, subsequent to the infection of nonneuronal CNS cells and especially of microglial cells. Infected microglial cells may play a role in the disease process by releasing not only virions or viral env-gene-encoded gp70 proteins but also other factors which may be directly or indirectly toxic to neurons. Parallels between microglial cell infection by MuLV and by lentiviruses, and specifically by human immunodeficiency virus, are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Andrews J. M., Gardner M. B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974 Apr;33(2):285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Baszler T. V., Zachary J. F. Murine retroviral neurovirulence correlates with an enhanced ability ofvirus to infect selectively, replicate in, and activate resident microglial cells. Am J Pathol. 1991 Mar;138(3):655–671. [PMC free article] [PubMed] [Google Scholar]
- Baszler T. V., Zachary J. F. Murine retroviral-induced spongiform neuronal degeneration parallels resident microglial cell infection: ultrastructural findings. Lab Invest. 1990 Nov;63(5):612–623. [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Contag C. H., Plagemann P. G. Age-dependent poliomyelitis of mice: expression of endogenous retrovirus correlates with cytocidal replication of lactate dehydrogenase-elevating virus in motor neurons. J Virol. 1989 Oct;63(10):4362–4369. doi: 10.1128/jvi.63.10.4362-4369.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Barrette M., Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol. 1984 Nov;52(2):356–363. doi: 10.1128/jvi.52.2.356-363.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Dow S. W., Poss M. L., Hoover E. A. Feline immunodeficiency virus: a neurotropic lentivirus. J Acquir Immune Defic Syndr. 1990;3(7):658–668. [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S. Efficient release of murine xenotropic oncornavirus after murine leukemia virus infection of mouse cells. Virology. 1975 May;65(1):304–307. doi: 10.1016/0042-6822(75)90036-7. [DOI] [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocyte-derived interleukin 3 as a growth factor for microglia cells and peritoneal macrophages. J Immunol. 1986 Dec 1;137(11):3521–3527. [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis. 1985 Jan-Feb;7(1):99–110. doi: 10.1093/clinids/7.1.99. [DOI] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Corpuz M. Brain glia release factors with opposing actions upon neuronal survival. J Neurosci. 1993 Jan;13(1):29–37. doi: 10.1523/JNEUROSCI.13-01-00029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Cimino E. F., Robbins D. S., Broadwell R. D., Powers J. M., Ruscetti S. K. Cellular tropism and localization in the rodent nervous system of a neuropathogenic variant of Friend murine leukemia virus. Lab Invest. 1992 Sep;67(3):314–321. [PubMed] [Google Scholar]
- Hoffman P. M., Pitts O. M., Bilello J. A., Cimino E. F. Retrovirus induced motor neuron degeneration. Rev Neurol (Paris) 1988;144(11):676–679. [PubMed] [Google Scholar]
- Huang M., Simard C., Jolicoeur P. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science. 1989 Dec 22;246(4937):1614–1617. doi: 10.1126/science.2480643. [DOI] [PubMed] [Google Scholar]
- Huang M., Simard C., Kay D. G., Jolicoeur P. The majority of cells infected with the defective murine AIDS virus belong to the B-cell lineage. J Virol. 1991 Dec;65(12):6562–6571. doi: 10.1128/jvi.65.12.6562-6571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E., DesGroseillers L., Robitaille Y., Paquette Y., Kay D. G. Retrovirus-induced motor neuron disease of mice: molecular basis of neurotropism and paralysis. Adv Neurol. 1991;56:481–493. [PubMed] [Google Scholar]
- Kay D. G., Gravel C., Pothier F., Laperrière A., Robitaille Y., Jolicoeur P. Neurological disease induced in transgenic mice expressing the env gene of the Cas-Br-E murine retrovirus. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4538–4542. doi: 10.1073/pnas.90.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
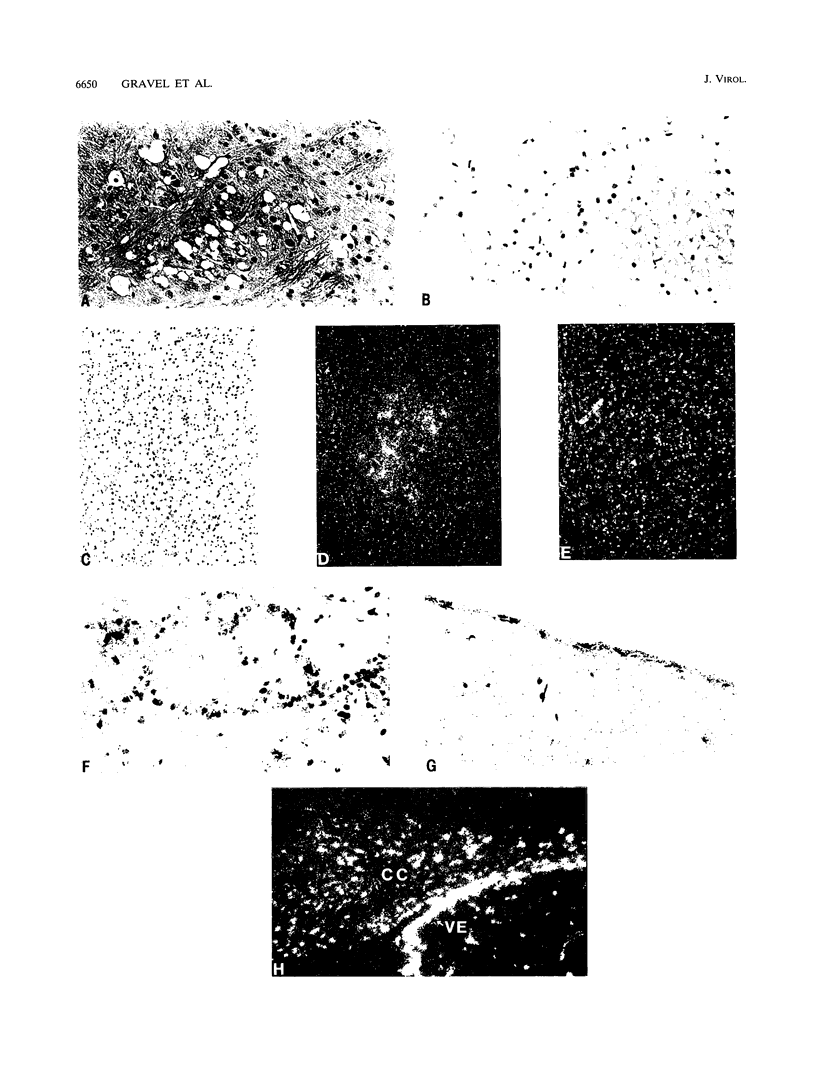
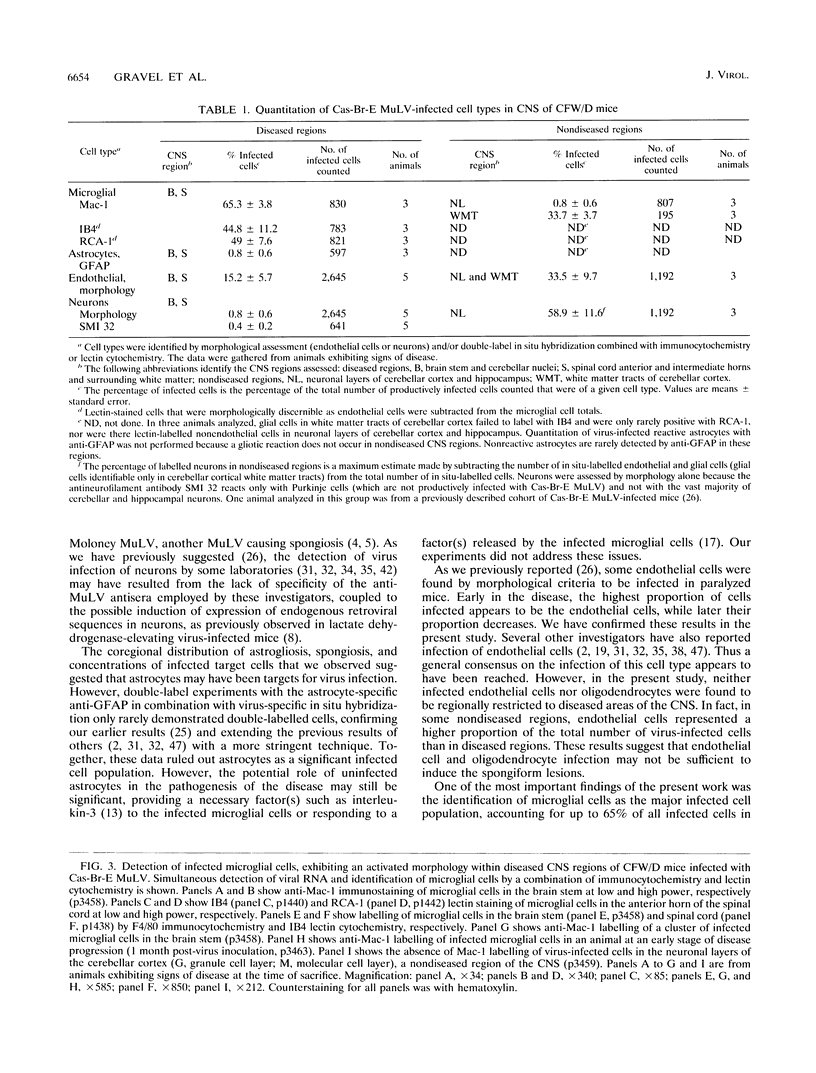
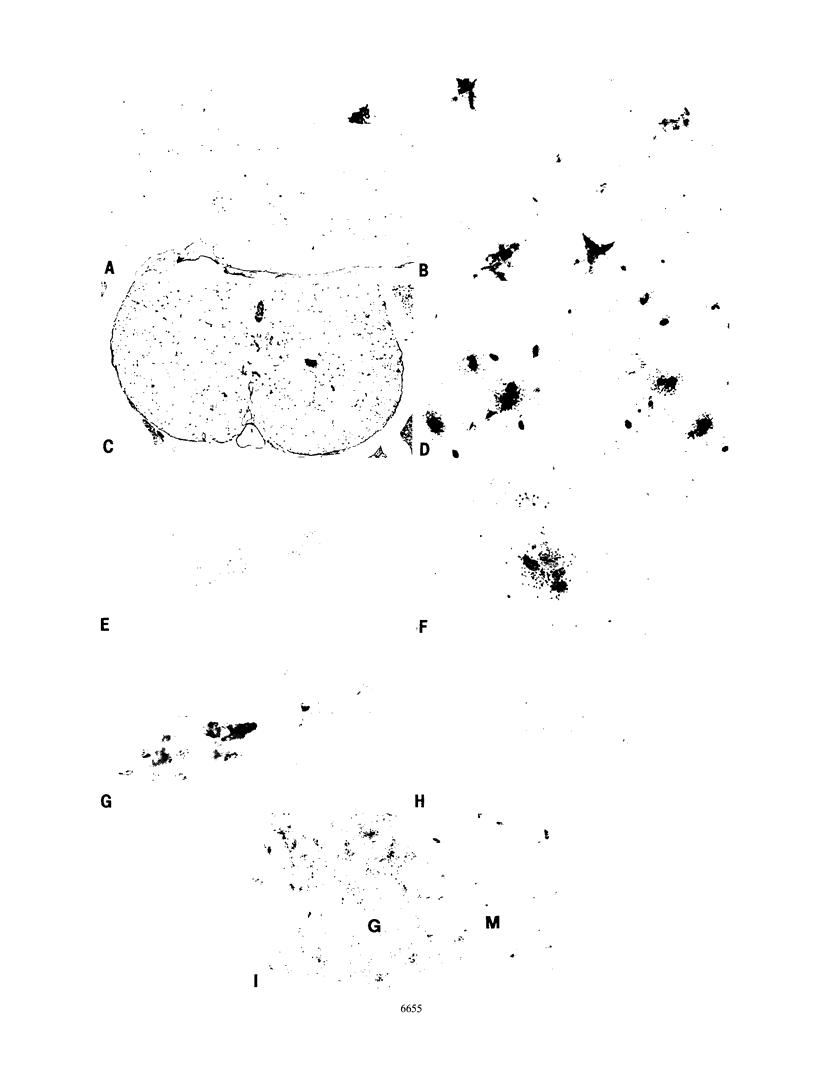
- Kay D. G., Gravel C., Robitaille Y., Jolicoeur P. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1281–1285. doi: 10.1073/pnas.88.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic type C viruses. Curr Top Microbiol Immunol. 1978;79:111–213. doi: 10.1007/978-3-642-66853-1_4. [DOI] [PubMed] [Google Scholar]
- Lynch W. P., Czub S., McAtee F. J., Hayes S. F., Portis J. L. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron. 1991 Sep;7(3):365–379. doi: 10.1016/0896-6273(91)90289-c. [DOI] [PubMed] [Google Scholar]
- Morey M. K., Wiley C. A. Immunohistochemical localization of neurotropic ecotropic murine leukemia virus in moribund mice. Virology. 1990 Sep;178(1):104–112. doi: 10.1016/0042-6822(90)90383-3. [DOI] [PubMed] [Google Scholar]
- Nagra R. M., Burrola P. G., Wiley C. A. Development of spongiform encephalopathy in retroviral infected mice. Lab Invest. 1992 Mar;66(3):292–302. [PubMed] [Google Scholar]
- Officer J. E., Tecson N., Estes J. D., Fontanilla E., Rongey R. W., Gardner M. B. Isolation of a neurotropic type C virus. Science. 1973 Sep 7;181(4103):945–947. doi: 10.1126/science.181.4103.945. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Jensen F., Dixon F. J., Lampert P. W. Pathogenesis of the slow disease of the central nervous system associated with wild mouse virus. II. Role of virus and host gene products. Virology. 1980 Nov;107(1):180–193. doi: 10.1016/0042-6822(80)90283-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Lampert P. W., Lee S., Dixon F. J. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am J Pathol. 1977 Jul;88(1):193–212. [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Kay D. G., Rassart E., Robitaille Y., Jolicoeur P. Substitution of the U3 long terminal repeat region of the neurotropic Cas-Br-E retrovirus affects its disease-inducing potential. J Virol. 1990 Aug;64(8):3742–3752. doi: 10.1128/jvi.64.8.3742-3752.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts O. M., Powers J. M., Bilello J. A., Hoffman P. M. Ultrastructural changes associated with retroviral replication in central nervous system capillary endothelial cells. Lab Invest. 1987 Apr;56(4):401–409. [PubMed] [Google Scholar]
- Portis J. L., Czub S., Garon C. F., McAtee F. J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990 Apr;64(4):1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Nelbach L., Jolicoeur P. Cas-Br-E murine leukemia virus: sequencing of the paralytogenic region of its genome and derivation of specific probes to study its origin and the structure of its recombinant genomes in leukemic tissues. J Virol. 1986 Dec;60(3):910–919. doi: 10.1128/jvi.60.3.910-919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Horowitz J. M., McCubrey J. Endogenous mouse leukemia viruses. Annu Rev Genet. 1983;17:85–121. doi: 10.1146/annurev.ge.17.120183.000505. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Hunter J. J., Chassler P., Jaenisch R. Role of abortive retroviral infection of neurons in spongiform CNS degeneration. Nature. 1990 Jul 12;346(6280):181–183. doi: 10.1038/346181a0. [DOI] [PubMed] [Google Scholar]
- Shikova E., Lin Y. C., Saha K., Brooks B. R., Wong P. K. Correlation of specific virus-astrocyte interactions and cytopathic effects induced by ts1, a neurovirulent mutant of Moloney murine leukemia virus. J Virol. 1993 Mar;67(3):1137–1147. doi: 10.1128/jvi.67.3.1137-1147.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard C., Jolicoeur P. The effect of anti-neoplastic drugs on murine acquired immunodeficiency syndrome. Science. 1991 Jan 18;251(4991):305–308. doi: 10.1126/science.1987646. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Swarz J. R., Brooks B. R., Johnson R. T. Spongiform polioencephalomyelopathy caused by a murine retrovirus. II. Ultrastructural localization of virus replication and spongiform changes in the central nervous system. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):365–380. doi: 10.1111/j.1365-2990.1981.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Wong P. K. Moloney murine leukemia virus temperature-sensitive mutants: a model for retrovirus-induced neurologic disorders. Curr Top Microbiol Immunol. 1990;160:29–60. doi: 10.1007/978-3-642-75267-4_3. [DOI] [PubMed] [Google Scholar]