Abstract
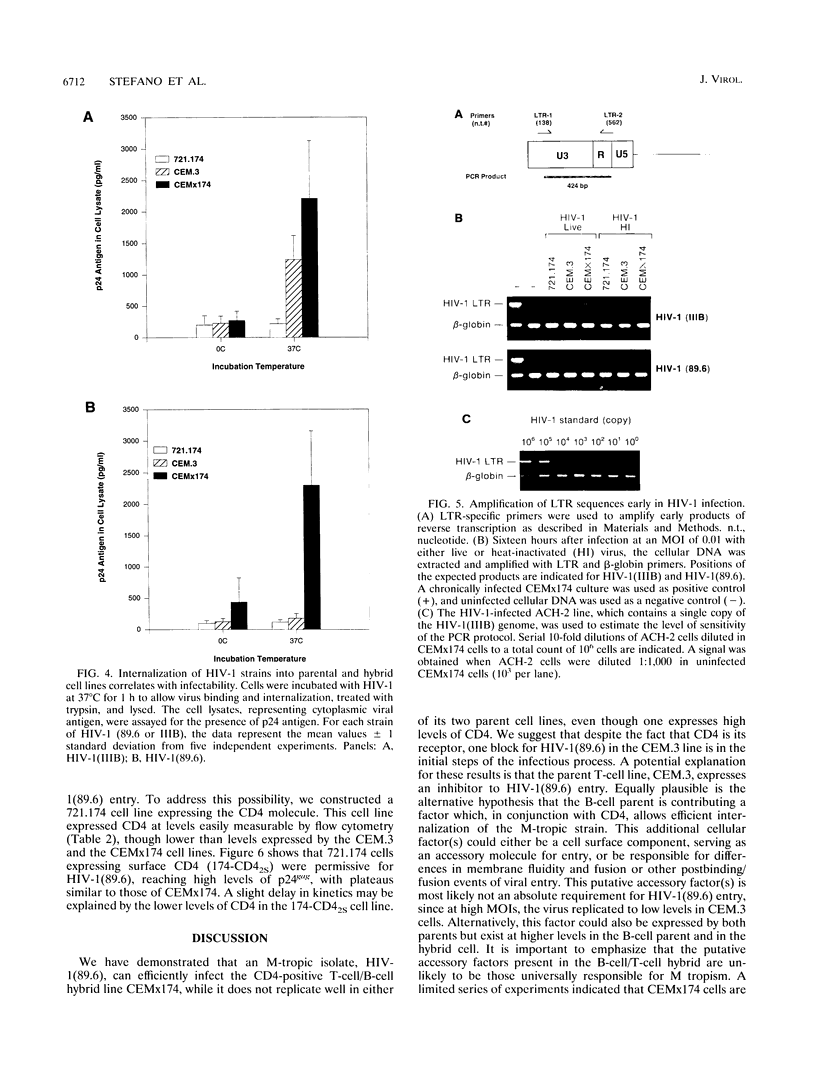
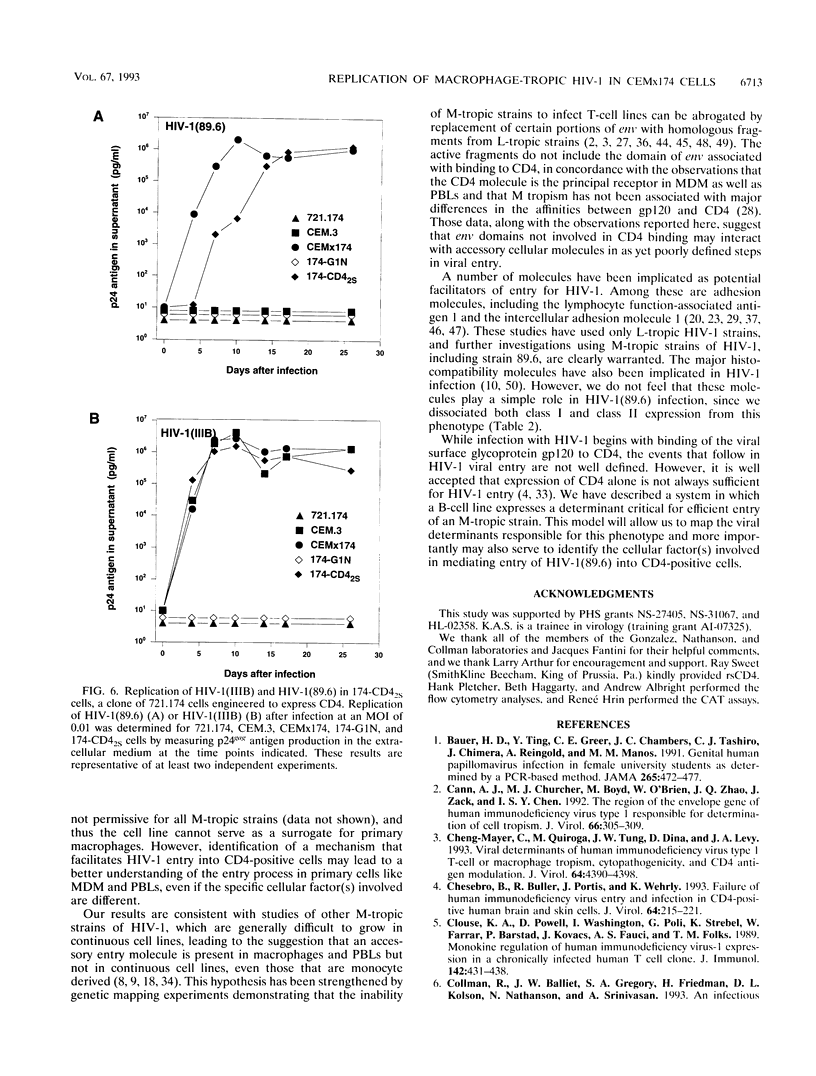
To investigate the mechanism underlying one aspect of the cellular tropism of human immunodeficiency virus type 1 (HIV-1), we used a macrophage-tropic isolate, 89.6, and screened its ability to infect a number of continuous cell lines. HIV-1 (89.6) was able to replicate robustly in a T-cell/B-cell hybrid line, CEMx174, while it replicated modestly or not at all in either of its parents, one of which is the CD4-positive line CEM.3. Analysis by transfection of a molecular clone, a virus uptake assay, and polymerase chain reaction all provided strong evidence that the block to HIV-1(89.6) replication in the CEM.3 line lies at the level of cellular entry. These results were complemented by preparing a CD4-expressing derivative of the B-cell parent, 721.174, and demonstrating that it is permissive for productive HIV-1(89.6) replication. Given these experimental findings, we speculate that there exist cellular accessory factors which facilitate virus entry and infection in CD4-positive cells. Furthermore, these cellular accessory factors may be quite virus strain specific, since not all macrophage-tropic strains of HIV-1 were able to replicate in the CEMx174 hybrid cell line. This experimental model provides a system for the identification of one or more of these putative cellular accessory factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H. M., Ting Y., Greer C. E., Chambers J. C., Tashiro C. J., Chimera J., Reingold A., Manos M. M. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991 Jan 23;265(4):472–477. [PubMed] [Google Scholar]
- Cann A. J., Churcher M. J., Boyd M., O'Brien W., Zhao J. Q., Zack J., Chen I. S. The region of the envelope gene of human immunodeficiency virus type 1 responsible for determination of cell tropism. J Virol. 1992 Jan;66(1):305–309. doi: 10.1128/jvi.66.1.305-309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Buller R., Portis J., Wehrly K. Failure of human immunodeficiency virus entry and infection in CD4-positive human brain and skin cells. J Virol. 1990 Jan;64(1):215–221. doi: 10.1128/jvi.64.1.215-221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Collman R., Godfrey B., Cutilli J., Rhodes A., Hassan N. F., Sweet R., Douglas S. D., Friedman H., Nathanson N., Gonzalez-Scarano F. Macrophage-tropic strains of human immunodeficiency virus type 1 utilize the CD4 receptor. J Virol. 1990 Sep;64(9):4468–4476. doi: 10.1128/jvi.64.9.4468-4476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman R., Hassan N. F., Walker R., Godfrey B., Cutilli J., Hastings J. C., Friedman H., Douglas S. D., Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989 Oct 1;170(4):1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Colizzi V. Role of major histocompatibility complex recognition in the protection and immunopathogenesis of AIDS. Joint Anglo-Italian Workshop on Ponza Island, Italy. AIDS. 1992 May;6(5):523–525. doi: 10.1097/00002030-199205000-00020. [DOI] [PubMed] [Google Scholar]
- DeMars R., Chang C. C., Shaw S., Reitnauer P. J., Sondel P. M. Homozygous deletions that simultaneously eliminate expressions of class I and class II antigens of EBV-transformed B-lymphoblastoid cells. I. Reduced proliferative responses of autologous and allogeneic T cells to mutant cells that have decreased expression of class II antigens. Hum Immunol. 1984 Oct;11(2):77–97. doi: 10.1016/0198-8859(84)90047-8. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Durda P. J., Leece B., Jenoski A., Rabin H., Fisher A., Wong-Staal F. Characterization of murine monoclonal antibodies to HIV-1 induced by synthetic peptides. AIDS Res Hum Retroviruses. 1988 Oct;4(5):331–342. doi: 10.1089/aid.1988.4.331. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T., Powell D. M., Lightfoote M. M., Benn S., Martin M. A., Fauci A. S. Induction of HTLV-III/LAV from a nonvirus-producing T-cell line: implications for latency. Science. 1986 Feb 7;231(4738):600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Betts R. F., Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1986 Nov 7;256(17):2365–2371. [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Husayni H., Turpin J. A., Skillman D., Kalter D. C., Orenstein J. M., Hoover D. L., Meltzer M. S. Macrophage-HIV interaction: viral isolation and target cell tropism. AIDS. 1990 Mar;4(3):221–228. [PubMed] [Google Scholar]
- Gruber M. F., Webb D. S., Gerrard T. L., Mostowski H. S., Vujcic L., Golding H. Re-evaluation of the involvement of the adhesion molecules ICAM-1/LFA-1 in syncytia formation of HIV-1-infected subclones of a CEM T-cell leukemic line. AIDS Res Hum Retroviruses. 1991 Jan;7(1):45–53. doi: 10.1089/aid.1991.7.45. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Bhat S., Spitalnik S. L., Laughlin M., Stefano K., Silberberg D. H., Gonzalez-Scarano F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991 Jul 19;253(5017):320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Laughlin M. A., Pletcher C., Friedman H. M., Gonzalez-Scarano F. Entry of human immunodeficiency virus-1 into glial cells proceeds via an alternate, efficient pathway. J Leukoc Biol. 1991 Jun;49(6):605–609. doi: 10.1002/jlb.49.6.605. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Orentas R. J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989 Jun 2;244(4908):1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D. N., Berger A. E., Cresswell P. Human T-B lymphoblast hybrids express HLA-DR specificities not expressed by either parent. Immunogenetics. 1982;15(2):199–206. doi: 10.1007/BF00621951. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Haggarty B. S., Bonser S. E., Rackowski J. L., Shan H., Kanki P. J. Biological characterization of a simian immunodeficiency virus-like retrovirus (HTLV-IV): evidence for CD4-associated molecules required for infection. J Virol. 1988 Aug;62(8):2557–2568. doi: 10.1128/jvi.62.8.2557-2568.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Culp J. S., Chaikin M. A., Hellmig B. D., Matthews T. J., Sweet R. W., Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter D. C., Gendelman H. E., Meltzer M. S. Inhibition of human immunodeficiency virus infection in monocytes by monoclonal antibodies against leukocyte adhesion molecules. Immunol Lett. 1991 Oct;30(2):219–227. doi: 10.1016/0165-2478(91)90029-a. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Ledbetter J. A., Kinney-Thomas E., Hu S. L. Effects of anti-gp120 monoclonal antibodies on CD4 receptor binding by the env protein of human immunodeficiency virus type 1. J Virol. 1988 Oct;62(10):3695–3702. doi: 10.1128/jvi.62.10.3695-3702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Poli G., Butini L., Fox C., Dayton A. I., Fauci A. S. Dissociation between syncytia formation and HIV spreading. Suppression of syncytia formation does not necessarily reflect inhibition of HIV infection. Eur J Immunol. 1991 Jul;21(7):1771–1774. doi: 10.1002/eji.1830210730. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Howell D. N., Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Dalgleish A. G., Weiss R. A., Beverley P. C. Epitopes of the CD4 antigen and HIV infection. Science. 1986 Nov 28;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Weiss R. A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988 Mar 11;52(5):631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N. E., O'Brien W. A., Verdin E., Kufta C. V., Chen I. S., Dubois-Dalcq M. Human immunodeficiency virus type 1 tropism for brain microglial cells is determined by a region of the env glycoprotein that also controls macrophage tropism. J Virol. 1992 Apr;66(4):2588–2593. doi: 10.1128/jvi.66.4.2588-2593.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Valentin A., Lundin K., Patarroyo M., Asjö B. The leukocyte adhesion glycoprotein CD18 participates in HIV-1-induced syncytia formation in monocytoid and T cells. J Immunol. 1990 Feb 1;144(3):934–937. [PubMed] [Google Scholar]
- Vermot-Desroches C., Rigal D., Escaich S., Bernaud J., Pichoud C., Lamelin J. P., Trepo C. Functional epitope analysis of the human CD11a/CD18 molecule (LFA-1, lymphocyte function-associated antigen 1) involved in HIV-1-induced syncytium formation. Scand J Immunol. 1991 Oct;34(4):461–470. doi: 10.1111/j.1365-3083.1991.tb01569.x. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Higgins D., Cheng-Mayer C., Bauer D., Levy J. A., Dina D. Human immunodeficiency virus type 1 cellular host range, replication, and cytopathicity are linked to the envelope region of the viral genome. J Virol. 1990 Aug;64(8):4016–4020. doi: 10.1128/jvi.64.8.4016-4020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A. HIV and HLA similarity. Nature. 1988 May 19;333(6170):215–215. doi: 10.1038/333215c0. [DOI] [PubMed] [Google Scholar]