Abstract
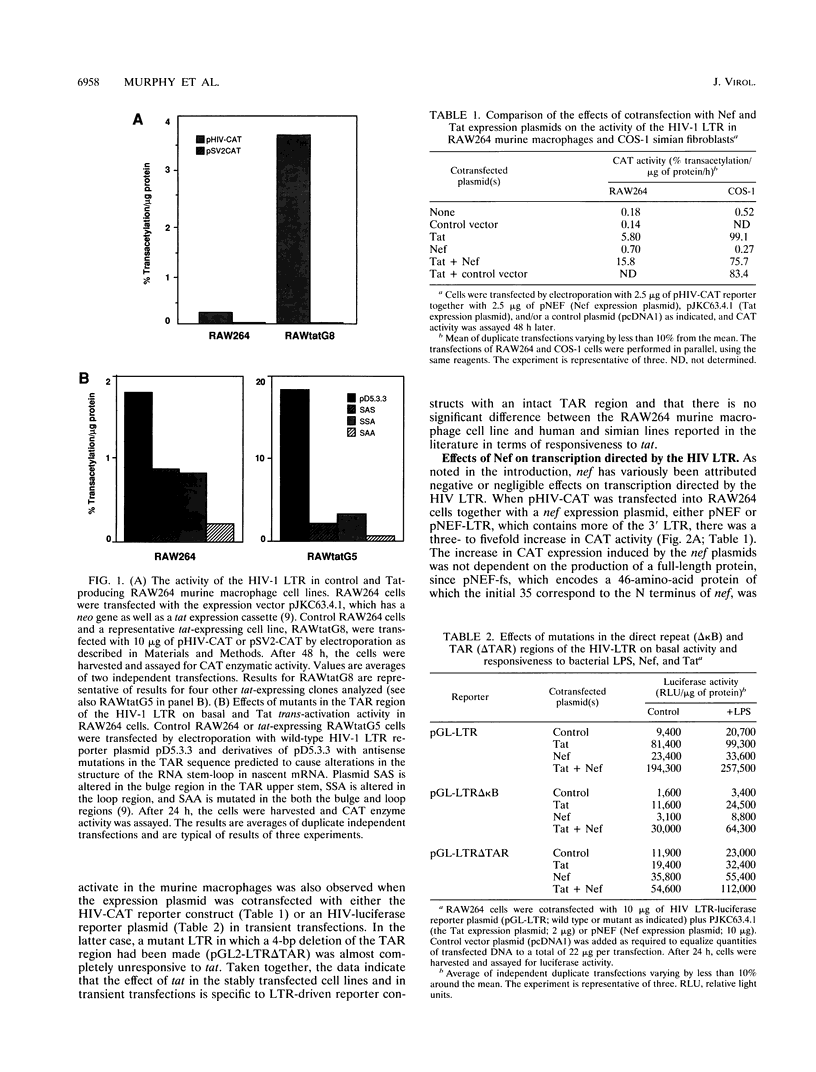
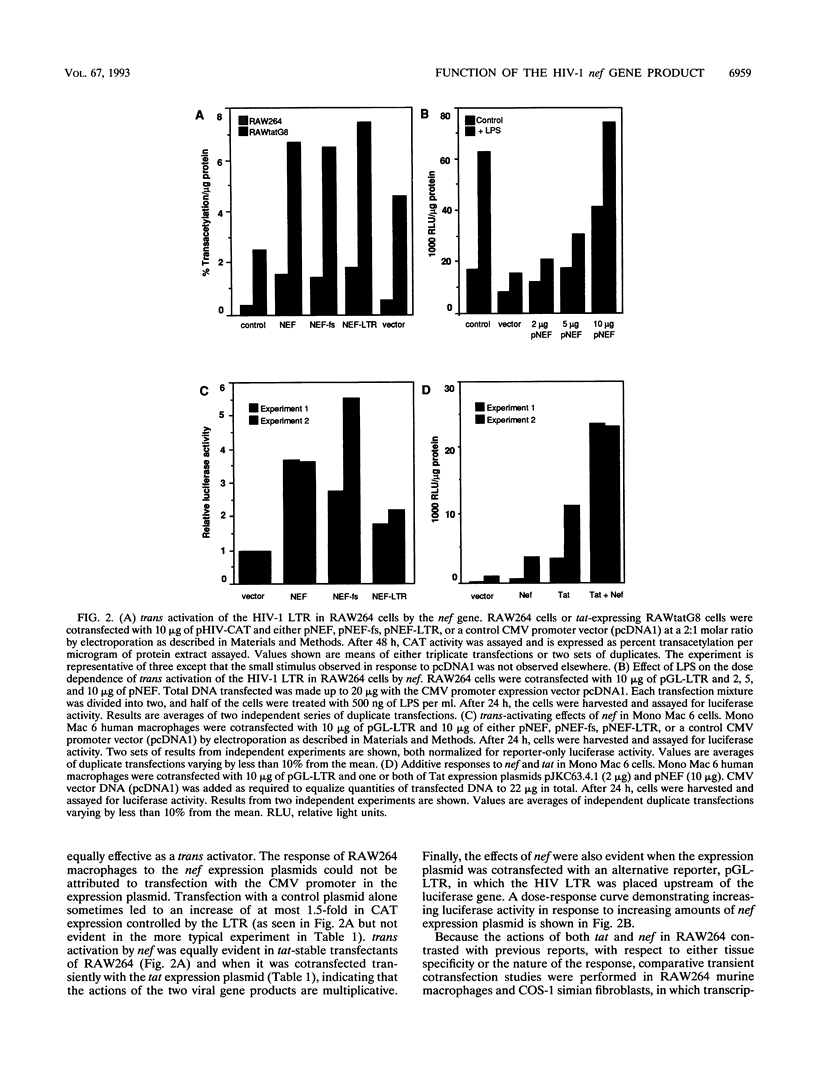
The RAW264 murine macrophage cell line was used as a model to examine the role of the tat and nef gene products in the transcription regulation of the human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) in macrophages. Contrary to claims that the activity of the HIV-1 LTR responds poorly in rodent cells to trans activation by the viral tat gene product, cotransfection of RAW264 cells with a tat expression plasmid in transient transfection assays caused a > 20-fold increase in reporter gene expression that was inhibited by mutations in the TAR region. RAW264 cells stably transfected with the tat plasmid displayed similarly elevated HIV-1 LTR-driven reporter gene activity. By contrast to previous reports indicating a negative role for nef in HIV transcription, cotransfection of RAW264 cells with a nef expression plasmid trans activated the HIV-1 LTR driving either a chloramphenicol acetyltransferase or a luciferase reporter gene. The action of nef was specific to the LTR, as expression of nef had no effect on the activity of the simian virus 40, c-fms, urokinase plasminogen activator, or type 5 acid phosphatase promoter. trans-activating activity was also manifested by a frameshift mutant expressing only the first 35 amino acids of the protein. The effects of nef were multiplicative with those of tat gene product and occurred even in the presence of bacterial lipopolysaccharide, which itself activated LTR-directed transcription. Examination of the effects of selected mutations in the LTR revealed that neither the kappa B sites in the direct repeat enhancer nor the TAR region was required as a cis-acting element in nef action. The action of nef was not species restricted; it was able to trans activate in the human monocyte-like cell line Mono Mac 6. The presence of a nef expression cassette in a neomycin phosphotransferase gene expression plasmid greatly reduced the number of G418-resistant colonies generated in stable transfection of RAW264 cells, and many of the colonies that were formed exhibited very slow growth. The frameshift mutant was also active in reducing colony generation. Given the absence of any effect of the frameshift mutation on nef function, its actions on macrophage growth and HIV transcription are discussed in terms of the role of the N-terminal 30 amino acids and of stable secondary structures in the mRNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Berkhout B., Jeang K. T. Functional roles for the TATA promoter and enhancers in basal and Tat-induced expression of the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1992 Jan;66(1):139–149. doi: 10.1128/jvi.66.1.139-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady A. I., Stacey K. J., Nimmo K. A., Murphy K. M., von der Ahe D., Pearson D., Botteri F. M., Nagamine Y., Hume D. A. Constitutive expression of the urokinase plasminogen activator gene in murine RAW264 macrophages involves distal and 5' non-coding sequences that are conserved between mouse and pig. Nucleic Acids Res. 1991 Dec 25;19(24):6839–6847. doi: 10.1093/nar/19.24.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corboy J. R., Buzy J. M., Zink M. C., Clements J. E. Expression directed from HIV long terminal repeats in the central nervous system of transgenic mice. Science. 1992 Dec 11;258(5089):1804–1808. doi: 10.1126/science.1465618. [DOI] [PubMed] [Google Scholar]
- Daniel-Issakani S., Spiegel A. M., Strulovici B. Lipopolysaccharide response is linked to the GTP binding protein, Gi2, in the promonocytic cell line U937. J Biol Chem. 1989 Dec 5;264(34):20240–20247. [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Desai K., Loewenstein P. M., Green M. Isolation of a cellular protein that binds to the human immunodeficiency virus Tat protein and can potentiate transactivation of the viral promoter. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8875–8879. doi: 10.1073/pnas.88.20.8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Ernberg I., Gait M. J., Green S. M., Heaphy S., Karn J., Lowe A. D., Singh M., Skinner M. A. HIV-1 tat protein stimulates transcription by binding to a U-rich bulge in the stem of the TAR RNA structure. EMBO J. 1990 Dec;9(12):4145–4153. doi: 10.1002/j.1460-2075.1990.tb07637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Robert-Guroff M., Ghrayeb J., Chang N. T., Wong-Staal F. Cytoplasmic localization of the HTLV-III 3' orf protein in cultured T cells. Virology. 1986 Dec;155(2):593–599. doi: 10.1016/0042-6822(86)90219-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy B., Kieny M. P., Riviere Y., Le Peuch C., Dott K., Girard M., Montagnier L., Lecocq J. P. HIV F/3' orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987 Nov 19;330(6145):266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Hammes S. R., Dixon E. P., Malim M. H., Cullen B. R., Greene W. C. Nef protein of human immunodeficiency virus type 1: evidence against its role as a transcriptional inhibitor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9549–9553. doi: 10.1073/pnas.86.23.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminchik J., Bashan N., Pinchasi D., Amit B., Sarver N., Johnston M. I., Fischer M., Yavin Z., Gorecki M., Panet A. Expression and biochemical characterization of human immunodeficiency virus type 1 nef gene product. J Virol. 1990 Jul;64(7):3447–3454. doi: 10.1128/jvi.64.7.3447-3454.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kim S., Ikeuchi K., Byrn R., Groopman J., Baltimore D. Lack of a negative influence on viral growth by the nef gene of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9544–9548. doi: 10.1073/pnas.86.23.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Fuerst T. R., Wood L. V., Woods R. M., Suzich J. A., Jones G. M., de la Cruz V. F., Davey R. T., Jr, Venkatesan S., Moss B. Mapping the fine specificity of a cytolytic T cell response to HIV-1 nef protein. J Immunol. 1990 Jul 1;145(1):127–135. [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Luria S., Chambers I., Berg P. Expression of the type 1 human immunodeficiency virus Nef protein in T cells prevents antigen receptor-mediated induction of interleukin 2 mRNA. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5326–5330. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Feinberg M. B., Trono D., Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990 Jul 1;172(1):253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Popovic M., Gartner S., Markham P., Gallo R. C., Reitz M. S. Structure and expression of tat-, rev-, and nef-specific transcripts of human immunodeficiency virus type 1 in infected lymphocytes and macrophages. J Virol. 1990 Jul;64(7):3391–3398. doi: 10.1128/jvi.64.7.3391-3398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Marciniak R. A. HIV TAR: an RNA enhancer? Cell. 1989 Oct 20;59(2):229–230. doi: 10.1016/0092-8674(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Irie K., Ninomiya-Tsuji J., Goebl M., Taniguchi T., Matsumoto K. New human gene encoding a positive modulator of HIV Tat-mediated transactivation. Nature. 1992 Jun 25;357(6380):700–702. doi: 10.1038/357700a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Szebeni J., Dieffenbach C., Wahl S. M., Venkateshan C. N., Yeh A., Popovic M., Gartner S., Wahl L. M., Peterfy M., Friedman R. M. Induction of alpha interferon by human immunodeficiency virus type 1 in human monocyte-macrophage cultures. J Virol. 1991 Nov;65(11):6362–6364. doi: 10.1128/jvi.65.11.6362-6364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger E., Sodroski J. G., Rosen C. A., Haseltine W. A. Effects of mutations within the 3' orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J Virol. 1986 Nov;60(2):754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav Y. N., Wong-Staal F. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- Yue X., Favot P., Dunn T. L., Cassady A. I., Hume D. A. Expression of mRNA encoding the macrophage colony-stimulating factor receptor (c-fms) is controlled by a constitutive promoter and tissue-specific transcription elongation. Mol Cell Biol. 1993 Jun;13(6):3191–3201. doi: 10.1128/mcb.13.6.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988 Mar 15;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]



