Abstract
To delineate the mechanisms of bovine leukemia virus (BLV) pathogenesis, four full-length BLV clones, 1, 8, 9, and 13, derived from the transformed cell line FLK-BLV and a clone construct, pBLV913, were introduced into bovine spleen cells by microinjection. Microinjected cells exhibited cytopathic effects and produced BLV p24 and gp51 antigens and infectious virus. The construct, pBLV913, was selected for infection of two sheep by inoculation of microinjected cells. After 15 months, peripheral blood mononuclear cells from these sheep served as inocula for the transfer of infection to four additional sheep. All six infected sheep seroconverted to BLV and had detectable BLV DNA in peripheral blood mononuclear cells after amplification by polymerase chain reaction. Four of the six sheep developed altered B/T-lymphocyte ratios between 33 and 53 months postinfection. One sheep died of unrelated causes, and one remained hematologically normal. Two of the affected sheep developed B lymphocytosis comparable to that observed in animals inoculated with peripheral blood mononuclear cells from BLV-infected cattle. This expanded B-lymphocyte population was characterized by elevated expression of B-cell surface markers, spontaneous blastogenesis, virus expression in vitro, and increased, polyclonally integrated provirus. One of these two sheep developed lymphocytic leukemia-lymphoma at 57 months postinfection. Leukemic cells had the same phenotype and harbored a single, monoclonally integrated provirus but produced no virus after in vitro cultivation. The range in clinical response to in vivo infection with cloned BLV suggests an important role for host immune response in the progression of virus replication and pathogenesis.
Full text
PDF




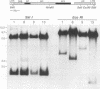
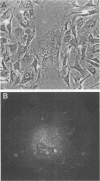
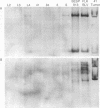
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Carpenter S., Christensen J., Storgaard T., Viuff B., Wannemuehler Y., Belousov J., Roth J. A. Identification of alternatively spliced mRNAs encoding potential new regulatory proteins in cattle infected with bovine leukemia virus. J Virol. 1993 Jan;67(1):39–52. doi: 10.1128/jvi.67.1.39-52.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga V., Ferrer J. F. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc Soc Exp Biol Med. 1977 Nov;156(2):388–391. doi: 10.3181/00379727-156-39942. [DOI] [PubMed] [Google Scholar]
- Boyd A. L., Wood T. G., Buckley A., Fischinger P. J., Gilden R. V., Gonda M. A. Microinjection and expression of an infectious proviral clone and subgenomic envelope construct of a human immunodeficiency virus. AIDS Res Hum Retroviruses. 1988 Feb;4(1):31–41. doi: 10.1089/aid.1988.4.31. [DOI] [PubMed] [Google Scholar]
- Brenner J., Van-Haam M., Savir D., Trainin Z. The implication of BLV infection in the productivity, reproductive capacity and survival rate of a dairy cow. Vet Immunol Immunopathol. 1989 Oct;22(3):299–305. doi: 10.1016/0165-2427(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Hoover E. A., LoBuglio A. F., Yon D. S. Phytomitogen- and antigen-induced blast transformation of feline lymphocytes. Am J Vet Res. 1975 Oct;36(10):1489–1494. [PubMed] [Google Scholar]
- Cockerell G. L., Rovnak J. The correlation between the direct and indirect detection of bovine leukemia virus infection in cattle. Leuk Res. 1988;12(6):465–469. doi: 10.1016/0145-2126(88)90112-9. [DOI] [PubMed] [Google Scholar]
- Derse D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J Virol. 1987 Aug;61(8):2462–2471. doi: 10.1128/jvi.61.8.2462-2471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Caradonna S. J., Casey J. W. Bovine leukemia virus long terminal repeat: a cell type-specific promoter. Science. 1985 Jan 18;227(4684):317–320. doi: 10.1126/science.2981431. [DOI] [PubMed] [Google Scholar]
- Derse D. trans-acting regulation of bovine leukemia virus mRNA processing. J Virol. 1988 Apr;62(4):1115–1119. doi: 10.1128/jvi.62.4.1115-1119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes L., Levy D., Parodi A. L., Levy J. P. Spontaneous immune response of bovine leukemia-virus-infected cattle against five different viral proteins. Int J Cancer. 1980 Apr 15;25(4):503–508. doi: 10.1002/ijc.2910250412. [DOI] [PubMed] [Google Scholar]
- Diacumakos E. G. Methods for micromanipulation of human somatic cells in culture. Methods Cell Biol. 1973;7:287–311. doi: 10.1016/s0091-679x(08)61783-5. [DOI] [PubMed] [Google Scholar]
- Dimmock C. K., Rogers R. J., Chung Y. S., McKenzie A. R., Waugh P. D. Differences in the lymphoproliferative response of cattle and sheep to bovine leucosis virus infection. Vet Immunol Immunopathol. 1986 Apr;11(4):325–331. doi: 10.1016/0165-2427(86)90035-8. [DOI] [PubMed] [Google Scholar]
- Dimmock C. K., Ward W. H., Trueman K. F. Lymphocyte subpopulations in sheep during the early stage of experimental infection with bovine leukaemia virus. Immunol Cell Biol. 1989 Apr;67(Pt 2):141–145. doi: 10.1038/icb.1989.20. [DOI] [PubMed] [Google Scholar]
- Djilali S., Parodi A. L., Levy D., Cockerell G. L. Development of leukemia and lymphosarcoma induced by bovine leukemia virus in sheep: a hematopathological study. Leukemia. 1987 Nov;1(11):777–781. [PubMed] [Google Scholar]
- Djilali S., Parodi A. L. The BLV-induced leukemia--lymphosarcoma complex in sheep. Vet Immunol Immunopathol. 1989 Oct;22(3):233–244. doi: 10.1016/0165-2427(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F. Bovine lymphosarcoma. Adv Vet Sci Comp Med. 1980;24:1–68. [PubMed] [Google Scholar]
- Ferrer J. F., Marshak R. R., Abt D. A., Kenyon S. J. Relationship between lymphosarcoma and persistent lymphocytosis in cattle: a review. J Am Vet Med Assoc. 1979 Oct 1;175(7):705–708. [PubMed] [Google Scholar]
- Fossum C., Burny A., Portetelle D., Mammerickx M., Morein B. Detection of B and T cells, with lectins or antibodies, in healthy and bovine leukemia virus-infected cattle. Vet Immunol Immunopathol. 1988 Apr;18(3):269–278. doi: 10.1016/0165-2427(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Bruck C., Kettmann R., Burny A. Bovine leukemia virus. Curr Top Microbiol Immunol. 1984;112:1–19. doi: 10.1007/978-3-642-69677-0_1. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Carter S. G., Kost T. A., Bess J. W., Jr, Arthur L. O., Van der Maaten M. J. Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. 1987 Nov 26-Dec 2Nature. 330(6146):388–391. doi: 10.1038/330388a0. [DOI] [PubMed] [Google Scholar]
- Haas L., Divers T., Casey J. W. Bovine leukemia virus gene expression in vivo. J Virol. 1992 Oct;66(10):6223–6225. doi: 10.1128/jvi.66.10.6223-6225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeney J. L., Valli P. J., Jacobs R. M., Valli V. E. Evidence for bovine leukemia virus infection of peripheral blood monocytes and limited antigen expression in bovine lymphoid tissue. Lab Invest. 1992 May;66(5):608–617. [PubMed] [Google Scholar]
- Heeney J. L., Valli V. E. Transformed phenotype of enzootic bovine lymphoma reflects differentiation-linked leukemogenesis. Lab Invest. 1990 Mar;62(3):339–346. [PubMed] [Google Scholar]
- Henning D., Nielsen K. Cross-reactivity of monoclonal antibodies to bovine immunoglobulins with immunoglobulins of other species. Vet Immunol Immunopathol. 1992 Nov;34(3-4):235–243. doi: 10.1016/0165-2427(92)90167-o. [DOI] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Ishino S., Matsuda I., Yamamoto H., Yoshino T., Sentsui H., Mizuno Y., Kono Y. Pathological findings of two types of lymphoid malignancy in sheep inoculated with bovine leukemia virus. Nihon Juigaku Zasshi. 1989 Aug;51(4):749–756. doi: 10.1292/jvms1939.51.749. [DOI] [PubMed] [Google Scholar]
- Itohara S., Sekikawa K. Molecular cloning of infectious proviral genomes of bovine leukemia virus. Virology. 1987 Jul;159(1):158–160. doi: 10.1016/0042-6822(87)90359-x. [DOI] [PubMed] [Google Scholar]
- Jensen W. A., Rovnak J., Cockerell G. L. In vivo transcription of the bovine leukemia virus tax/rex region in normal and neoplastic lymphocytes of cattle and sheep. J Virol. 1991 May;65(5):2484–2490. doi: 10.1128/jvi.65.5.2484-2490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Cleuter Y., Mammerickx M., Meunier-Rotival M., Bernardi G., Burny A., Chantrenne H. Genomic integration of bovine leukemia provirus: comparison of persistent lymphocytosis with lymph node tumor form of enzootic. Proc Natl Acad Sci U S A. 1980 May;77(5):2577–2581. doi: 10.1073/pnas.77.5.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Kumar S. P., Paul P. S., Pomeroy K. A., Johnson D. W., Muscoplat C. C., Van Der Maaten M. J., Miller J. M., Sorensen D. K. Frequency of lymphocytes bearing Fc receptors and surface membrane immunoglobulins in normal, persistent lymphocytotic and leukemia cows. Am J Vet Res. 1978 Jan;39(1):45–49. [PubMed] [Google Scholar]
- Lagarias D. M., Radke K. Transcriptional activation of bovine leukemia virus in blood cells from experimentally infected, asymptomatic sheep with latent infections. J Virol. 1989 May;63(5):2099–2107. doi: 10.1128/jvi.63.5.2099-2107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D., Deshayes L., Parodi A. L., Levy J. P., Stephenson J. R., Devare S. G., Gilden R. V. Bovine leukemia virus specific antibodies among French cattle. II. Radioimmunoassay with the major structural protein (BLV p24). Int J Cancer. 1977 Oct 15;20(4):543–550. doi: 10.1002/ijc.2910200411. [DOI] [PubMed] [Google Scholar]
- Lewin H. A., Wu M. C., Stewart J. A., Nolan T. J. Association between BoLA and subclinical bovine leukemia virus infection in a herd of Holstein-Friesian cows. Immunogenetics. 1988;27(5):338–344. doi: 10.1007/BF00395129. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Gogolin-Ewens K. J., Brandon M. R. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology. 1985 Aug;55(4):729–737. [PMC free article] [PubMed] [Google Scholar]
- Mamoun R. Z., Morisson M., Rebeyrotte N., Busetta B., Couez D., Kettmann R., Hospital M., Guillemain B. Sequence variability of bovine leukemia virus env gene and its relevance to the structure and antigenicity of the glycoproteins. J Virol. 1990 Sep;64(9):4180–4188. doi: 10.1128/jvi.64.9.4180-4188.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheise J. P., Delcommenne M., Mager A., Didembourg C. H., Letesson J. J. CD5+ B cells from bovine leukemia virus infected cows are activated cycling cells responsive to interleukin 2. Leukemia. 1992 Apr;6(4):304–309. [PubMed] [Google Scholar]
- Muscoplat C. C., Alhaji I., Johnson D. W., Pomeroy K. A., Olson J. M., Larson V. L., Stevens J. B., Sorensen D. K. Characteristics of lymphocyte responses to phytomitogens: comparison of responses of lymphocytes from normal and lymphocytotic cows. Am J Vet Res. 1974 Aug;35(8):1053–1055. [PubMed] [Google Scholar]
- Portetelle D., Mammerickx M., Burny A. Use of two monoclonal antibodies in an ELISA test for the detection of antibodies to bovine leukaemia virus envelope protein gp51. J Virol Methods. 1989 Feb;23(2):211–222. doi: 10.1016/0166-0934(89)90135-3. [DOI] [PubMed] [Google Scholar]
- Powers M. A., Grossman D., Kidd L. C., Radke K. Episodic occurrence of antibodies against the bovine leukemia virus Rex protein during the course of infection in sheep. J Virol. 1991 Sep;65(9):4959–4965. doi: 10.1128/jvi.65.9.4959-4965.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N. K., Mackay C. R., Brandon M. R. Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules. Immunology. 1985 Dec;56(4):725–733. [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Burny A., Gilden R. V. The gag and pol genes of bovine leukemia virus: nucleotide sequence and analysis. Virology. 1985 Apr 30;142(2):357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Couez D., Deschamps J., Kettmann R., Burny A., Gilden R. V. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology. 1984 Oct 15;138(1):82–93. doi: 10.1016/0042-6822(84)90149-1. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Burny A., Haseltine W. A. Trans activation of the bovine leukemia virus long terminal repeat in BLV-infected cells. Science. 1985 Jan 18;227(4684):320–322. doi: 10.1126/science.2981432. [DOI] [PubMed] [Google Scholar]
- Rosenthal S., Drescher B., Weber S., Möhring R. Nucleotide sequence analysis of the 3' half of the genome of bovine leukaemia virus grown in FLK cells. Acta Virol. 1990 Feb;34(1):1–10. [PubMed] [Google Scholar]
- Rovnak J., Casey J. W., Boyd A. L., Gonda M. A., Cockerell G. L. Isolation of bovine leukemia virus infected endothelial cells from cattle with persistent lymphocytosis. Lab Invest. 1991 Aug;65(2):192–202. [PubMed] [Google Scholar]
- Sagata N., Tsuzuku-Kawamura J., Nagayoshi-Aida M., Shimizu F., Imagawa K., Ikawa Y. Identification and some biochemical properties of the major XBL gene product of bovine leukemia virus. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7879–7883. doi: 10.1073/pnas.82.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima I., Olson C. Effect of mitogens and anti-bovine leukosis virus serums on DNA synthesis of lymphocytes from cattle. Eur J Cancer. 1980 May;16(5):639–645. doi: 10.1016/0014-2964(80)90204-2. [DOI] [PubMed] [Google Scholar]
- Van den Broeke A., Cleuter Y., Chen G., Portetelle D., Mammerickx M., Zagury D., Fouchard M., Coulombel L., Kettmann R., Burny A. Even transcriptionally competent proviruses are silent in bovine leukemia virus-induced sheep tumor cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9263–9267. doi: 10.1073/pnas.85.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. H., Dimmock C. K., Eaves F. W. T lymphocyte responses of sheep to bovine leukaemia virus infection. Immunol Cell Biol. 1992 Oct;70(Pt 5):329–336. doi: 10.1038/icb.1992.42. [DOI] [PubMed] [Google Scholar]
- Willems L., Kettmann R., Dequiedt F., Portetelle D., Vonèche V., Cornil I., Kerkhofs P., Burny A., Mammerickx M. In vivo infection of sheep by bovine leukemia virus mutants. J Virol. 1993 Jul;67(7):4078–4085. doi: 10.1128/jvi.67.7.4078-4085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems L., Portetelle D., Kerkhofs P., Chen G., Burny A., Mammerickx M., Kettmann R. In vivo transfection of bovine leukemia provirus into sheep. Virology. 1992 Aug;189(2):775–777. doi: 10.1016/0042-6822(92)90604-n. [DOI] [PubMed] [Google Scholar]
- Willems L., Thienpont E., Kerkhofs P., Burny A., Mammerickx M., Kettmann R. Bovine leukemia virus, an animal model for the study of intrastrain variability. J Virol. 1993 Feb;67(2):1086–1089. doi: 10.1128/jvi.67.2.1086-1089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. J., Hare W. C., Abt D. A. DNA and RNA synthesis in cultures of peripheral blood mononuclear leucocytes from normal and lymphosarcomatous cows. Br J Haematol. 1967 Nov;13(6):855–861. doi: 10.1111/j.1365-2141.1967.tb08856.x. [DOI] [PubMed] [Google Scholar]