Abstract
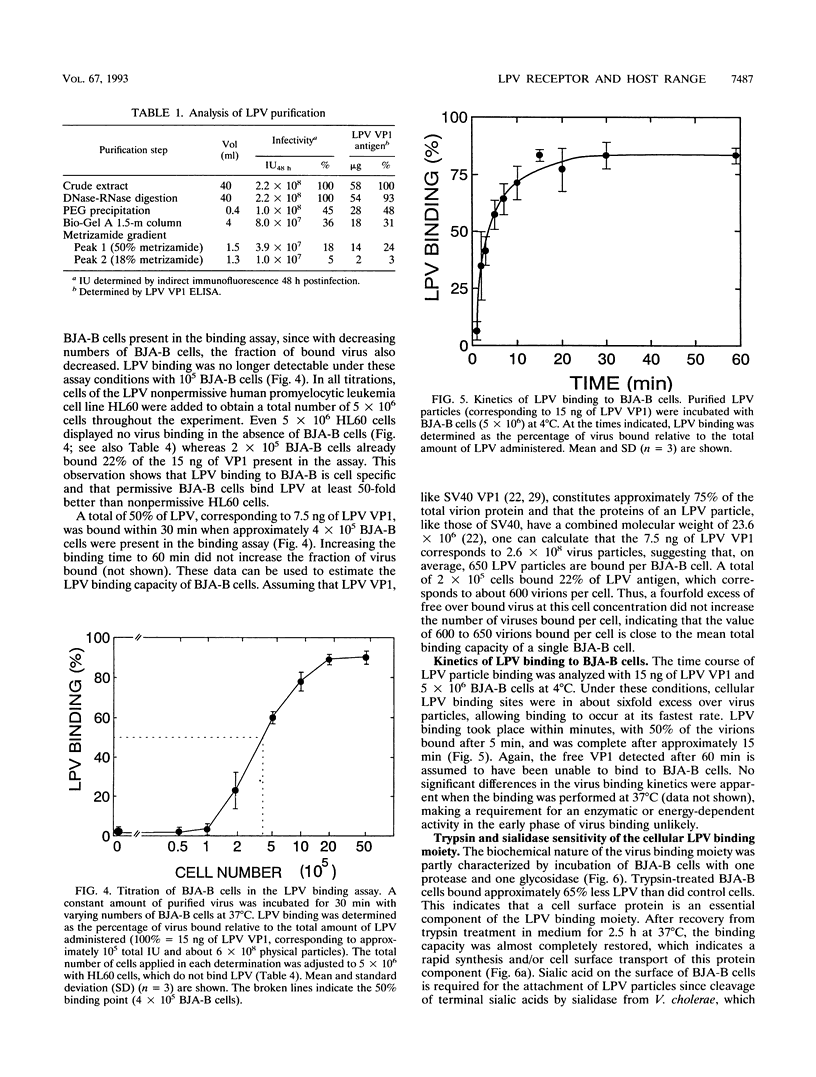
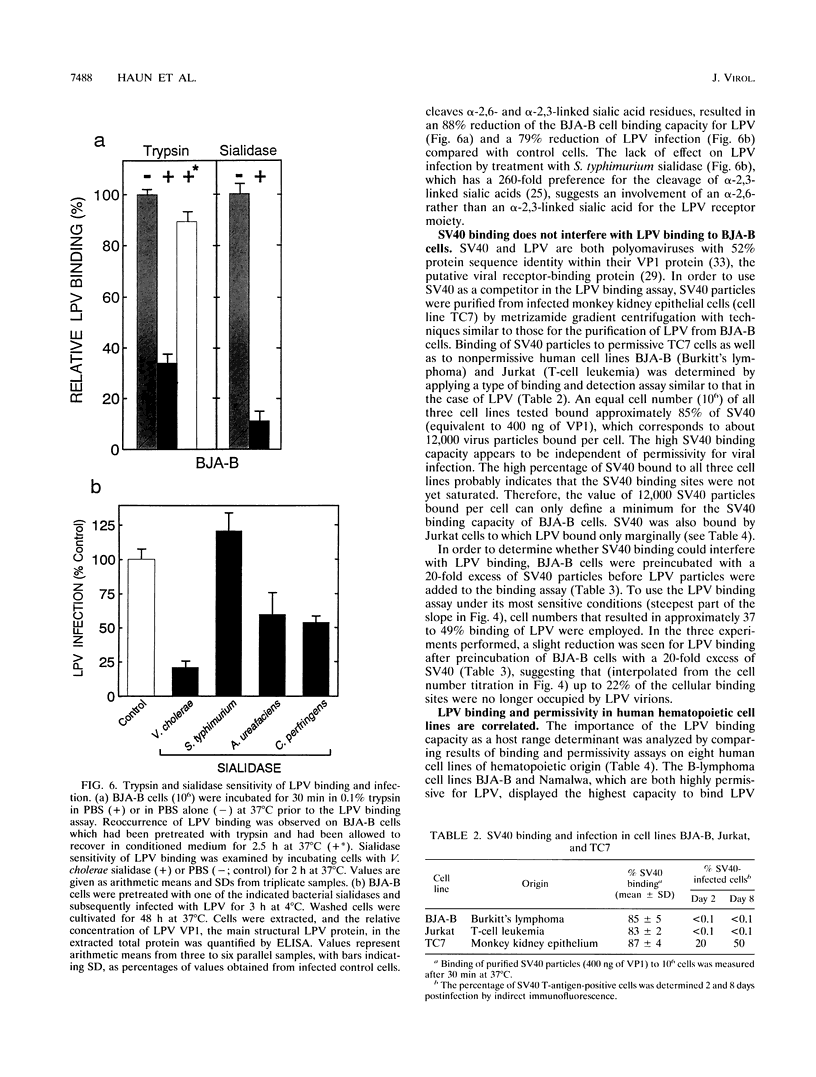
The B-lymphotropic papovavirus (LPV) productively infects only a subset of human B-lymphoma-derived cell lines while transfection of the viral genome yields infectious viral particles in a much wider variety of human hematopoietic cell lines. We have analyzed the contribution of a putative LPV receptor on the cell surface of B-cell lines in restricting the virus host range. In order to establish a quantitative virus binding assay for LPV, infectious virus particles were highly purified by metrizamide equilibrium density centrifugation and used as immunogens to raise seven mouse monoclonal antibodies specific for LPV VP1. Virus particle binding was quantitated in an indirect, nonradioactive assay with an LPV VP1-specific enzyme-linked immunosorbent assay. Binding of LPV particles to permissive human B-lymphoma cell line BJA-B occurred within minutes. Kinetics and capacity of binding were similar at 4 and 37 degrees C. A BJA-B cell was estimated to bind approximately 600 virus particles at conditions under which 50% of the administered virus was bound. The sialidase and trypsin sensitivities of the cellular virus binding moiety show that sialylated and proteinaceous components are necessary components of the LPV receptor on BJA-B cells. Despite a high binding capacity of BJA-B cells for simian virus 40, LPV binding was not significantly affected by a 20-fold excess of simian virus 40 particles, indicating that these related polyomaviruses do not bind to the same receptor on BJA-B cells. Reduction of LPV binding to sialidase-pretreated BJA-B cells was accompanied by a similar reduction of infection, indicating that virus binding may be a limiting factor in the LPV replicative cycle. The two highly LPV-permissive human B-lymphoma cell lines BJA-B and Namalwa displayed high virus binding whereas low and nonpermissive hematopoietic cell lines showed reduced or undetectable virus binding. We conclude that the inability of LPV particles to productively infect the nonpermissive human hematopoietic cell lines analyzed is probably due to the absence or insufficient expression of a functional cell surface receptor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Oldstone M. B. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J Virol. 1992 Dec;66(12):7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Vogl W., Gissman L., zur Hausen H. Propagation of B-lymphotropic papovavirus (LPV) in human B-lymphoma cells and characterization of its DNA. Virology. 1981 Oct 15;114(1):228–235. doi: 10.1016/0042-6822(81)90268-3. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. The adsorption of polyoma virus. Virology. 1962 Oct;18:177–181. doi: 10.1016/0042-6822(62)90003-x. [DOI] [PubMed] [Google Scholar]
- Cahan L. D., Singh R., Paulson J. C. Sialyloligosaccharide receptors of binding variants of polyoma virus. Virology. 1983 Oct 30;130(2):281–289. doi: 10.1016/0042-6822(83)90083-1. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Neilson K., Van Dyke T. Lymphotropic papovavirus early region is specifically regulated transgenic mice and efficiently induces neoplasia. J Virol. 1989 May;63(5):2204–2214. doi: 10.1128/jvi.63.5.2204-2214.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Brando L. V., Compans R. W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989 May;63(5):2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Compans R. W. Characterization of simian virus 40 receptor moieties on the surfaces of Vero C1008 cells. J Virol. 1989 Mar;63(3):1095–1100. doi: 10.1128/jvi.63.3.1095-1100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Compans R. W. Entry of simian virus 40 is restricted to apical surfaces of polarized epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3391–3396. doi: 10.1128/mcb.8.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L. K., Sjöström H., Norén O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992 Jun 4;357(6377):417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Forbes B., Gissmann L., Pawlita M. Detection of individual virus-infected cells by filter in situ hybridization. Mol Cell Probes. 1988 Sep;2(3):245–253. doi: 10.1016/0890-8508(88)90008-4. [DOI] [PubMed] [Google Scholar]
- Fried H., Cahan L. D., Paulson J. C. Polyoma virus recognizes specific sialyligosaccharide receptors on host cells. Virology. 1981 Feb;109(1):188–192. doi: 10.1016/0042-6822(81)90485-2. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Hoyer L. L., Roggentin P., Schauer R., Vimr E. R. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl alpha 2----3 linkages. J Biochem. 1991 Sep;110(3):462–467. doi: 10.1093/oxfordjournals.jbchem.a123603. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenoir G. M., Vuillaume M., Bonnardel C. The use of lymphomatous and lymphoblastoid cell lines in the study of Burkitt's lymphoma. IARC Sci Publ. 1985;(60):309–318. [PubMed] [Google Scholar]
- Liddington R. C., Yan Y., Moulai J., Sahli R., Benjamin T. L., Harrison S. C. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991 Nov 28;354(6351):278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Mosthaf L., Pawlita M., Gruss P. A viral enhancer element specifically active in human haematopoietic cells. Nature. 1985 Jun 13;315(6020):597–600. doi: 10.1038/315597a0. [DOI] [PubMed] [Google Scholar]
- Pawlita M., Clad A., zur Hausen H. Complete DNA sequence of lymphotropic papovavirus: prototype of a new species of the polyomavirus genus. Virology. 1985 May;143(1):196–211. doi: 10.1016/0042-6822(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Pawlita M., Mosthaf L., Clad A., Gruss P. Genome structure and host range restriction of the lymphotropic papovavirus (LPV): identification of a viral lymphocyte specific enhancer element. Curr Top Microbiol Immunol. 1984;113:26–30. doi: 10.1007/978-3-642-69860-6_5. [DOI] [PubMed] [Google Scholar]
- Pawlita M., Mushinski E., Feldmann R. J., Potter M. A monoclonal antibody that defines an idiotope with two subsites in galactan-binding myeloma proteins. J Exp Med. 1981 Dec 1;154(6):1946–1956. doi: 10.1084/jem.154.6.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Birnie G. D. Metrizamide, a new density-gradient medium. FEBS Lett. 1975 Feb 1;50(2):102–110. doi: 10.1016/0014-5793(75)80467-4. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Schneider U., Schwenk H. U., Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977 May 15;19(5):621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Szajnert M. F., Saule S., Bornkamm G. W., Wajcman H., Lenoir G. M., Kaplan J. C. Clustered somatic mutations in and around first exon of non-rearranged c-myc in Burkitt lymphoma with t(8;22) translocation. Nucleic Acids Res. 1987 Jun 11;15(11):4553–4565. doi: 10.1093/nar/15.11.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Furuno A., Kato K., Yoshiike K. Biological and biochemical studies of African green monkey lymphotropic papovavirus. J Virol. 1982 May;42(2):502–509. doi: 10.1128/jvi.42.2.502-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Kanda T. Lymphotropic papovavirus transformation of hamster embryo cells. J Virol. 1984 Apr;50(1):100–105. doi: 10.1128/jvi.50.1.100-105.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini J. E., Graham D., DeWitt C. M., Lineberger D. W., Rodkey J. A., Colonno R. J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E. M., King G. L., Maratos-Flier E. Characterization of a common high-affinity receptor for reovirus serotypes 1 and 3 on endothelial cells. J Virol. 1989 Mar;63(3):1318–1325. doi: 10.1128/jvi.63.3.1318-1325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Möller P., Moldenhauer G., Schirrmacher V., Pawlita M., Wolf J. Local growth of a Burkitt's lymphoma versus disseminated invasive growth of the autologous EBV-immortalized lymphoblastoid cells and their somatic cell hybrids in SCID mice. Int J Cancer. 1992 Jan 21;50(2):265–273. doi: 10.1002/ijc.2910500217. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979 Aug;167(3):137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]