Abstract
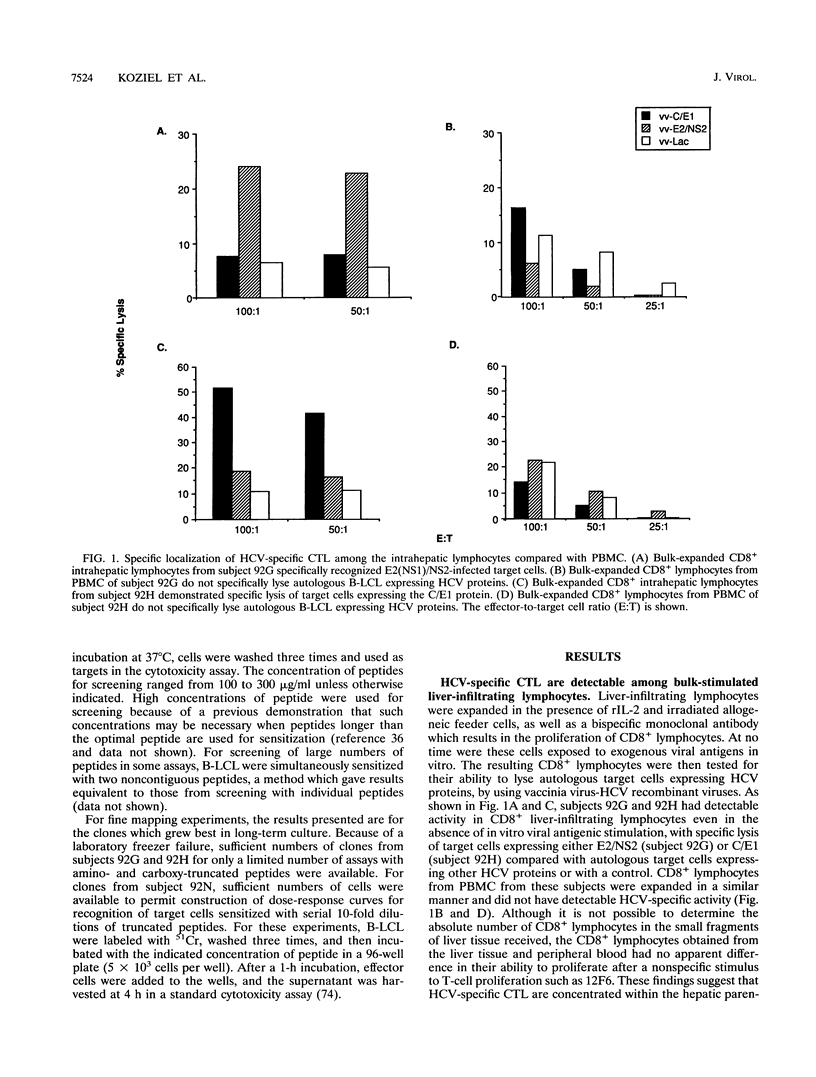
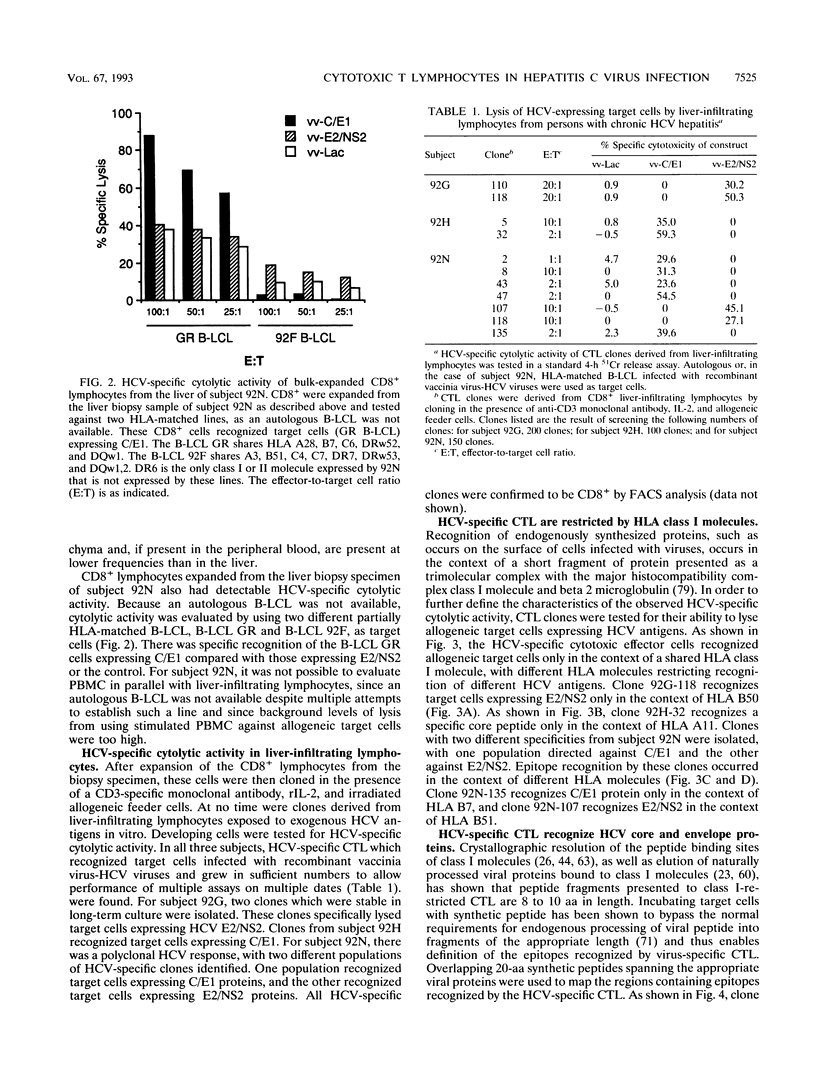
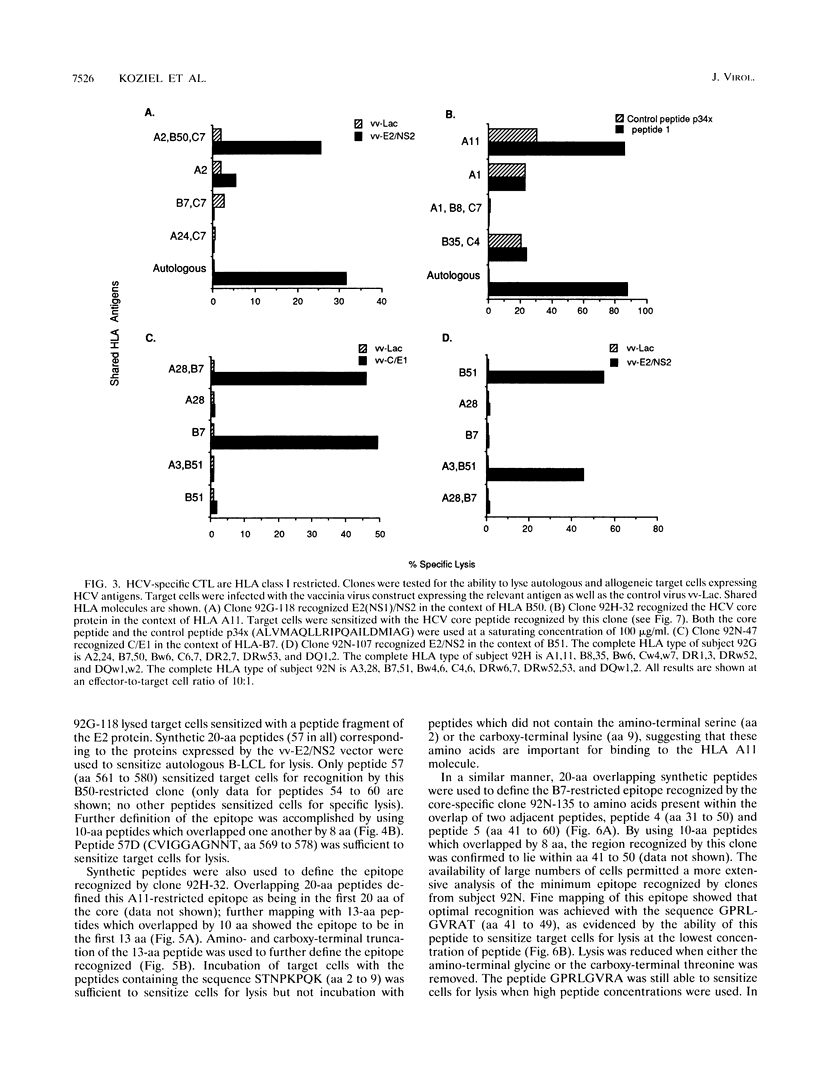
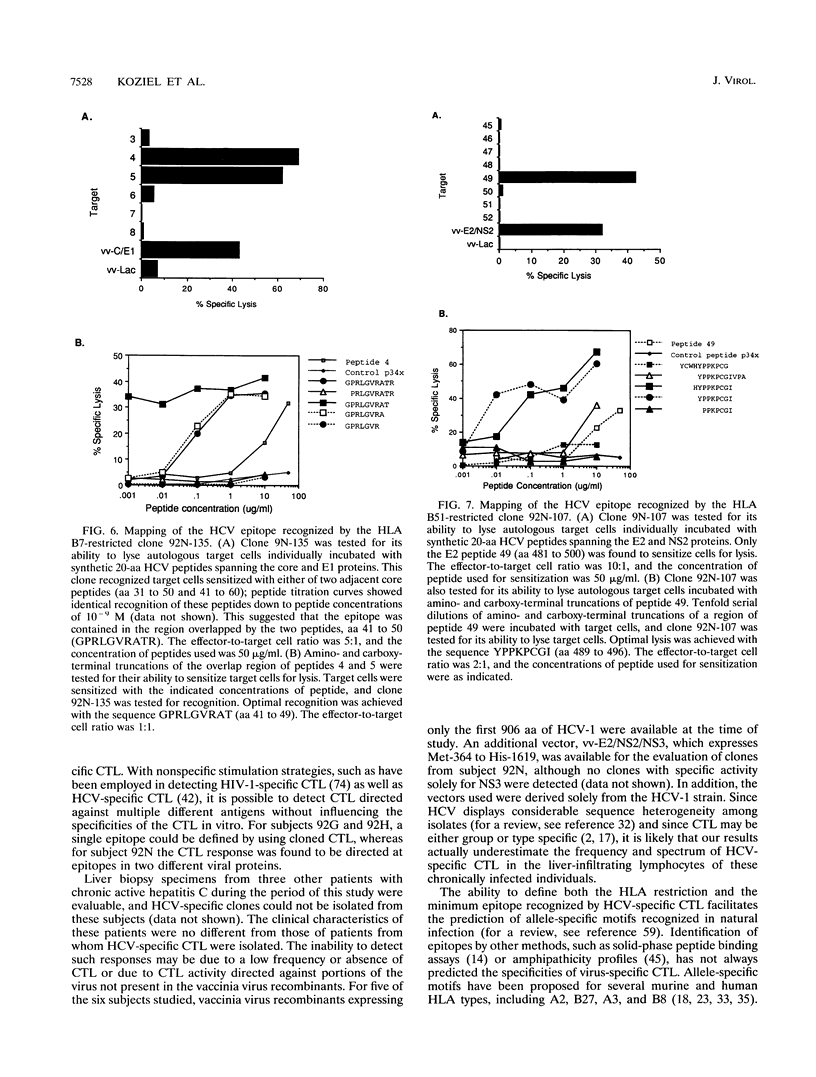
Hepatitis C virus (HCV) is a major cause of posttransfusion and community-acquired hepatitis, and a majority of individuals infected with this virus will subsequently develop chronic hepatitis. Characterization of the host immune response to this infection is an important first step that should facilitate the development of immunomodulatory agents and vaccines. Cellular immune responses, especially those mediated by cytotoxic T lymphocytes (CTL), are important in the control of many viral diseases. In this study, liver-infiltrating lymphocytes from persons with chronic HCV hepatitis were examined for evidence of HCV-specific CTL by using target cells infected with recombinant vaccinia viruses expressing the HCV core, E1, E2, and part of the NS2 proteins. Bulk expansion of liver-derived CD8+ lymphocytes resulted in the detection of HCV-specific CTL activity, whereas activity could not be found in CD8+ lymphocytes expanded from peripheral blood. Epitopes recognized by these CTL were defined by using CTL clones obtained by limiting dilution and target cells sensitized with synthetic HCV peptides. Four distinct HLA class I-restricted epitopes were identified, including two epitopes in the amino-terminal portion of the core protein. These studies provide evidence that the highly conserved core protein is a target for HCV-specific CTL and identify CTL epitopes within the more highly variable E2 envelope protein. Our studies also suggest that HCV-specific CTL are localized at the site of tissue injury in infected persons with chronic hepatitis. Identification of the epitopes recognized by HCV-specific CTL will facilitate exploration of their role in disease pathogenesis and may provide information useful in development of therapeutic interventions or vaccines.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Inchauspe G., Shikata T., Prince A. M. Three different patterns of hepatitis C virus infection in chimpanzees. Hepatology. 1992 Apr;15(4):690–695. doi: 10.1002/hep.1840150423. [DOI] [PubMed] [Google Scholar]
- Aebischer T., Moskophidis D., Rohrer U. H., Zinkernagel R. M., Hengartner H. In vitro selection of lymphocytic choriomeningitis virus escape mutants by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11047–11051. doi: 10.1073/pnas.88.24.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Alter M. J., Margolis H. S., Krawczynski K., Judson F. N., Mares A., Alexander W. J., Hu P. Y., Miller J. K., Gerber M. A., Sampliner R. E. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992 Dec 31;327(27):1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- Bodmer J. G., Marsh S. G., Albert E. D., Bodmer W. F., Dupont B., Erlich H. A., Mach B., Mayr W. R., Parham P., Sasazuki T. Nomenclature for factors of the HLA system, 1991. Hum Immunol. 1992 May;34(1):4–18. doi: 10.1016/0198-8859(92)90079-3. [DOI] [PubMed] [Google Scholar]
- Bortolotti F., Tagger A., Cadrobbi P., Crivellaro C., Pregliasco F., Ribero M. L., Alberti A. Antibodies to hepatitis C virus in community-acquired acute non-A, non-B hepatitis. J Hepatol. 1991 Mar;12(2):176–180. doi: 10.1016/0168-8278(91)90935-5. [DOI] [PubMed] [Google Scholar]
- Botarelli P., Brunetto M. R., Minutello M. A., Calvo P., Unutmaz D., Weiner A. J., Choo Q. L., Shuster J. R., Kuo G., Bonino F. T-lymphocyte response to hepatitis C virus in different clinical courses of infection. Gastroenterology. 1993 Feb;104(2):580–587. doi: 10.1016/0016-5085(93)90430-k. [DOI] [PubMed] [Google Scholar]
- Cha T. A., Beall E., Irvine B., Kolberg J., Chien D., Kuo G., Urdea M. S. At least five related, but distinct, hepatitis C viral genotypes exist. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7144–7148. doi: 10.1073/pnas.89.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin J., Martinon F., Connan F., Gomard E., Levy J. P. HLA-binding regions of HIV-1 proteins. I. Detection of seven HLA binding regions in the HIV-1 Nef protein. J Immunol. 1991 Jul 15;147(2):569–574. [PubMed] [Google Scholar]
- Culmann B., Gomard E., Kiény M. P., Guy B., Dreyfus F., Saimot A. G., Sereni D., Sicard D., Lévy J. P. Six epitopes reacting with human cytotoxic CD8+ T cells in the central region of the HIV-1 NEF protein. J Immunol. 1991 Mar 1;146(5):1560–1565. [PubMed] [Google Scholar]
- DiBrino M., Parker K. C., Shiloach J., Knierman M., Lukszo J., Turner R. V., Biddison W. E., Coligan J. E. Endogenous peptides bound to HLA-A3 possess a specific combination of anchor residues that permit identification of potential antigenic peptides. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1508–1512. doi: 10.1073/pnas.90.4.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag J. L. Non-A, non-B hepatitis. I. Recognition, epidemiology, and clinical features. Gastroenterology. 1983 Aug;85(2):439–462. [PubMed] [Google Scholar]
- Dodd R. Y. The risk of transfusion-transmitted infection. N Engl J Med. 1992 Aug 6;327(6):419–421. doi: 10.1056/NEJM199208063270610. [DOI] [PubMed] [Google Scholar]
- Eckart M. R., Selby M., Masiarz F., Lee C., Berger K., Crawford K., Kuo C., Kuo G., Houghton M., Choo Q. L. The hepatitis C virus encodes a serine protease involved in processing of the putative nonstructural proteins from the viral polyprotein precursor. Biochem Biophys Res Commun. 1993 Apr 30;192(2):399–406. doi: 10.1006/bbrc.1993.1429. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Govindarajan S., Wong D. C., Engle R., Lesniewski R. R., Mushahwar I. K., Desai S. M., Miller R. H., Ogata N. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992 Oct 2;258(5079):135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Wong D., Miller R. H., Shih J. W., Jett B., Purcell R. H. A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med. 1991 Jul 11;325(2):98–104. doi: 10.1056/NEJM199107113250205. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Gavioli R., Kurilla M. G., de Campos-Lima P. O., Wallace L. E., Dolcetti R., Murray R. J., Rickinson A. B., Masucci M. G. Multiple HLA A11-restricted cytotoxic T-lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J Virol. 1993 Mar;67(3):1572–1578. doi: 10.1128/jvi.67.3.1572-1578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles P. N., Guerrette D. L., Ulevitch R. J., Schreiber R. D., Chisari F. V. HBsAg retention sensitizes the hepatocyte to injury by physiological concentrations of interferon-gamma. Hepatology. 1992 Sep;16(3):655–663. doi: 10.1002/hep.1840160308. [DOI] [PubMed] [Google Scholar]
- Grakoui A., McCourt D. W., Wychowski C., Feinstone S. M., Rice C. M. Characterization of the hepatitis C virus-encoded serine proteinase: determination of proteinase-dependent polyprotein cleavage sites. J Virol. 1993 May;67(5):2832–2843. doi: 10.1128/jvi.67.5.2832-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Wychowski C., Lin C., Feinstone S. M., Rice C. M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993 Mar;67(3):1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Kato N., Ootsuyama Y., Nakagawa M., Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Weiner A., Han J., Kuo G., Choo Q. L. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991 Aug;14(2):381–388. [PubMed] [Google Scholar]
- Jelachich M. L., Cowan E. P., Turner R. V., Coligan J. E., Biddison W. E. Analysis of the molecular basis of HLA-A3 recognition by cytotoxic T cells using defined mutants of the HLA-A3 molecule. J Immunol. 1988 Aug 15;141(4):1108–1113. [PubMed] [Google Scholar]
- Jin Y., Shih W. K., Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988 Jul 1;168(1):293–306. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. P., Trocha A., Buchanan T. M., Walker B. D. Identification of overlapping HLA class I-restricted cytotoxic T cell epitopes in a conserved region of the human immunodeficiency virus type 1 envelope glycoprotein: definition of minimum epitopes and analysis of the effects of sequence variation. J Exp Med. 1992 Apr 1;175(4):961–971. doi: 10.1084/jem.175.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. P., Trocha A., Yang L., Mazzara G. P., Panicali D. L., Buchanan T. M., Walker B. D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991 Sep 1;147(5):1512–1521. [PubMed] [Google Scholar]
- Johnson R. P., Walker B. D. Identification of HIV-1 cytotoxic T-lymphocyte epitopes and implications for vaccine development. Biotechnol Ther. 1991;2(1-2):137–146. [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L. S., Whitton J. L., Oldstone M. B. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J Virol. 1989 Oct;63(10):4311–4316. doi: 10.1128/jvi.63.10.4311-4316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Fuerst T. R., Wood L. V., Woods R. M., Suzich J. A., Jones G. M., de la Cruz V. F., Davey R. T., Jr, Venkatesan S., Moss B. Mapping the fine specificity of a cytolytic T cell response to HIV-1 nef protein. J Immunol. 1990 Jul 1;145(1):127–135. [PubMed] [Google Scholar]
- Koziel M. J., Dudley D., Wong J. T., Dienstag J., Houghton M., Ralston R., Walker B. D. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992 Nov 15;149(10):3339–3344. [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Martell M., Esteban J. I., Quer J., Genescà J., Weiner A., Esteban R., Guardia J., Gómez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992 May;66(5):3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmish F., Chan S. W., Dow B. C., Gillon J., Frame W. D., Crawford R. J., Yap P. L., Follett E. A., Simmonds P. Detection of three types of hepatitis C virus in blood donors: investigation of type-specific differences in serologic reactivity and rate of alanine aminotransferase abnormalities. Transfusion. 1993 Jan;33(1):7–13. doi: 10.1046/j.1537-2995.1993.33193142314.x. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli M., Eddleston A. L. Mechanisms of liver cell injury in acute and chronic hepatitis B. Semin Liver Dis. 1984 Feb;4(1):47–58. doi: 10.1055/s-2008-1040645. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C. A., Palmiter R. D., Brinster R. L., Kanagawa O., Chisari F. V. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990 Apr 20;248(4953):361–364. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- Nasoff M. S., Zebedee S. L., Inchauspé G., Prince A. M. Identification of an immunodominant epitope within the capsid protein of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5462–5466. doi: 10.1073/pnas.88.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B., Eppler M., Leist T. P., Hengartner H., Zinkernagel R. M. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen S., Hengartner H., Zinkernagel R. M. Vaccination for disease. Science. 1991 Jan 11;251(4990):195–198. doi: 10.1126/science.1824801. [DOI] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack M. S. Cytotoxic T-lymphocytes. Adv Intern Med. 1988;33:17–44. [PubMed] [Google Scholar]
- Peters M., Vierling J., Gershwin M. E., Milich D., Chisari F. V., Hoofnagle J. H. Immunology and the liver. Hepatology. 1991 May;13(5):977–994. [PubMed] [Google Scholar]
- Phillips R. E., Rowland-Jones S., Nixon D. F., Gotch F. M., Edwards J. P., Ogunlesi A. O., Elvin J. G., Rothbard J. A., Bangham C. R., Rizza C. R. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991 Dec 12;354(6353):453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Brotman B., Huima T., Pascual D., Jaffery M., Inchauspé G. Immunity in hepatitis C infection. J Infect Dis. 1992 Mar;165(3):438–443. doi: 10.1093/infdis/165.3.438. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K. Naturally-occurring peptide antigens derived from the MHC class-I-restricted processing pathway. Immunol Today. 1991 Dec;12(12):447–455. doi: 10.1016/0167-5699(91)90018-O. [DOI] [PubMed] [Google Scholar]
- Saito I., Miyamura T., Ohbayashi A., Harada H., Katayama T., Kikuchi S., Watanabe Y., Koi S., Onji M., Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Schild H., Deres K., Wiesmüller K. H., Jung G., Rammensee H. G. Efficiency of peptides and lipopeptides for in vivo priming of virus-specific cytotoxic T cells. Eur J Immunol. 1991 Nov;21(11):2649–2654. doi: 10.1002/eji.1830211102. [DOI] [PubMed] [Google Scholar]
- Seeff L. B., Buskell-Bales Z., Wright E. C., Durako S. J., Alter H. J., Iber F. L., Hollinger F. B., Gitnick G., Knodell R. G., Perrillo R. P. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992 Dec 31;327(27):1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- Selby M. J., Choo Q. L., Berger K., Kuo G., Glazer E., Eckart M., Lee C., Chien D., Kuo C., Houghton M. Expression, identification and subcellular localization of the proteins encoded by the hepatitis C viral genome. J Gen Virol. 1993 Jun;74(Pt 6):1103–1113. doi: 10.1099/0022-1317-74-6-1103. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Alexander D., Rugroden M. E., Choo Q. L., Berger K., Crawford K., Kuo C., Leng S., Lee C., Ralston R. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology. 1992 Jun;188(2):819–830. doi: 10.1016/0042-6822(92)90537-y. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Wahren B., Magnius L. O. Immune response to a single peptide containing an immunodominant region of hepatitis C virus core protein: the isotypes and the recognition site. Immunol Lett. 1992 Jun;33(1):27–33. doi: 10.1016/0165-2478(92)90089-7. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Merli S., Putney S. D., Houghten R., Moss B., Germain R. N., Berzofsky J. A. A single amino acid interchange yields reciprocal CTL specificities for HIV-1 gp160. Science. 1989 Oct 6;246(4926):118–121. doi: 10.1126/science.2789433. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Boonmar S., Kubo Y., Katayama T., Harada H., Ohbayashi A., Choo Q. L., Kuo G., Houghton M., Saito I. Hepatitis C viral cDNA clones isolated from a healthy carrier donor implicated in post-transfusion non-A, non-B hepatitis. Gene. 1990 Jul 16;91(2):287–291. doi: 10.1016/0378-1119(90)90102-w. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Venet A., Walker B. D. Cytotoxic T-cell epitopes in HIV/SIV infection. AIDS. 1993;7 (Suppl 1):S117–S126. [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Birch-Limberger K., Fisher L., Paradis T. J., Aldovini A., Young R., Moss B., Schooley R. T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. J., Brauer M. J., Rosenblatt J., Richman K. H., Tung J., Crawford K., Bonino F., Saracco G., Choo Q. L., Houghton M. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991 Feb;180(2):842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- Wong J. T., Colvin R. B. Bi-specific monoclonal antibodies: selective binding and complement fixation to cells that express two different surface antigens. J Immunol. 1987 Aug 15;139(4):1369–1374. [PubMed] [Google Scholar]
- Wong J. T., Pinto C. E., Gifford J. D., Kurnick J. T., Kradin R. L. Characterization of the CD4+ and CD8+ tumor infiltrating lymphocytes propagated with bispecific monoclonal antibodies. J Immunol. 1989 Nov 15;143(10):3404–3411. [PubMed] [Google Scholar]
- Zhang Q. J., Gavioli R., Klein G., Masucci M. G. An HLA-A11-specific motif in nonamer peptides derived from viral and cellular proteins. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2217–2221. doi: 10.1073/pnas.90.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Haenseler E., Leist T., Cerny A., Hengartner H., Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986 Oct 1;164(4):1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos-Lima P. O., Gavioli R., Zhang Q. J., Wallace L. E., Dolcetti R., Rowe M., Rickinson A. B., Masucci M. G. HLA-A11 epitope loss isolates of Epstein-Barr virus from a highly A11+ population. Science. 1993 Apr 2;260(5104):98–100. doi: 10.1126/science.7682013. [DOI] [PubMed] [Google Scholar]


