Abstract
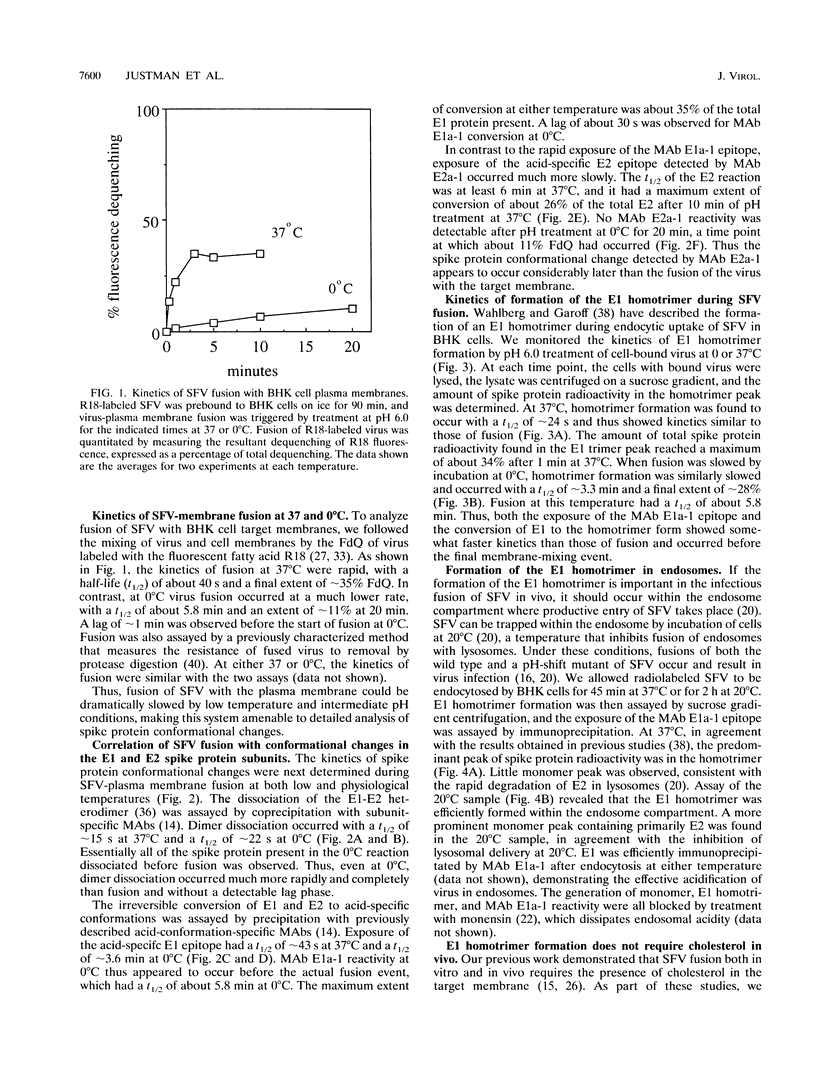
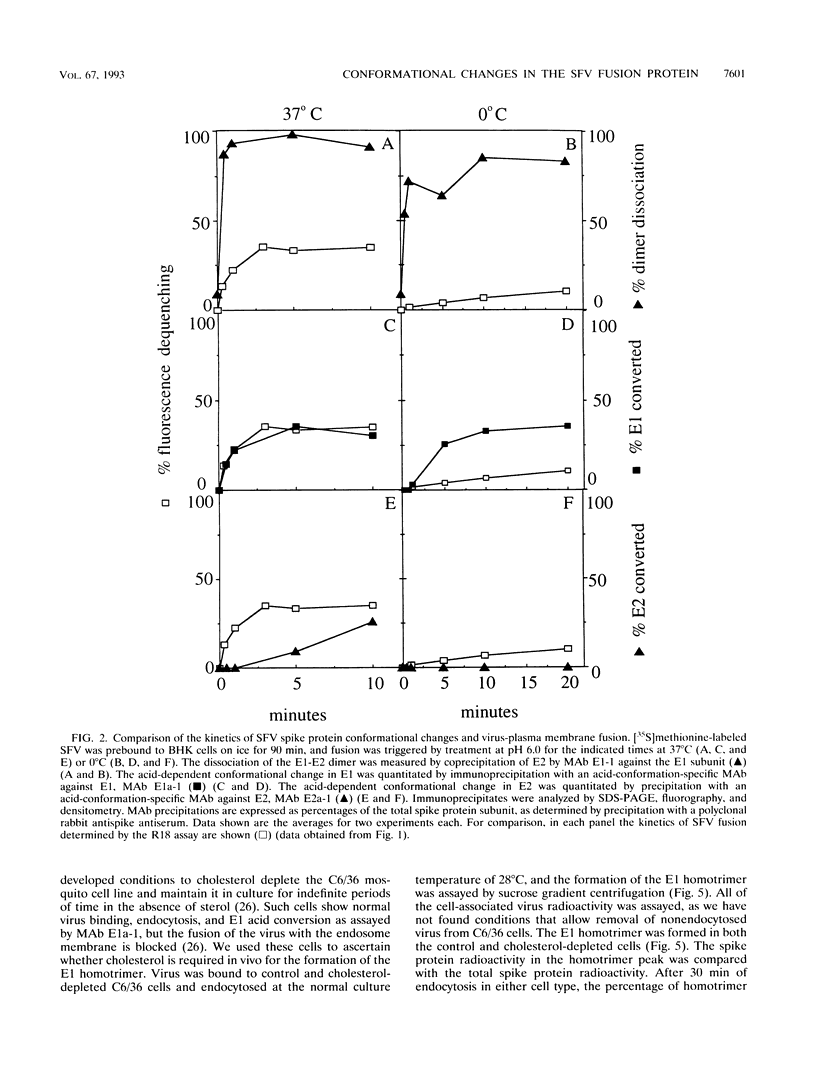
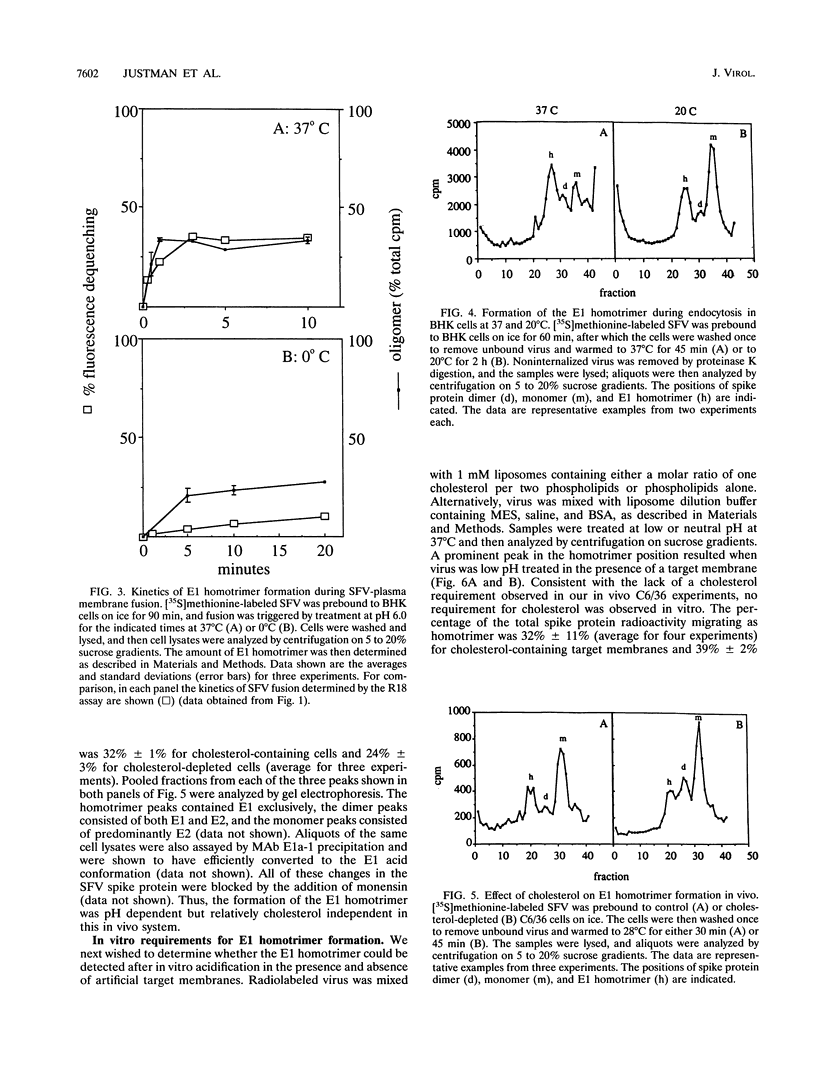
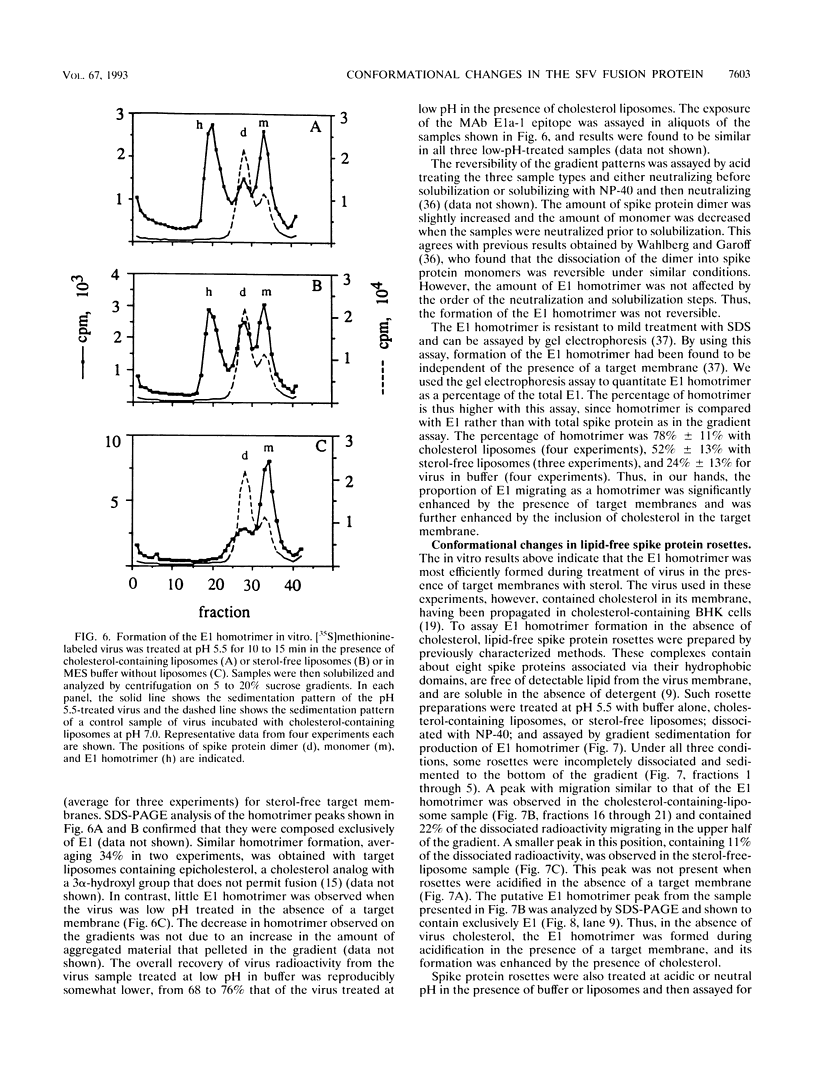
The alphavirus Semliki Forest virus (SFV) and a number of other enveloped animal viruses infect cells via a membrane fusion reaction triggered by the low pH within endocytic vesicles. In addition to having a low pH requirement, SFV fusion and infection are also strictly dependent on the presence of cholesterol in the host cell membrane. A number of conformational changes in the SFV spike protein occur following low-pH treatment, including dissociation of the E1-E2 dimer, conformational changes in the E1 and E2 subunits, and oligomerization of E1 to a homotrimer. To allow the ordering of these events, we have compared the kinetics of these conformational changes with those of fusion, using pH treatment near the fusion threshold and low-temperature incubation to slow the fusion reaction. Dimer dissociation, the E1 conformational change, and E1 trimerization all occur prior to the mixing of virus and cell membranes. Studies of cells incubated at 20 degrees C showed that as with virus fusion, E1 trimerization occurred in the endosome before transport to lysosomes. However, unlike the strictly cholesterol-dependent membrane fusion reaction, the E1 homotrimer was produced in vivo during virus uptake by cholesterol-depleted cells or in vitro by low-pH treatment of virus in the presence of artificial liposomes with or without cholesterol. Purified, lipid-free spike protein rosettes were assayed to determine the requirement for virus membrane cholesterol in E1 homotrimer formation. Spike protein rosettes were found to undergo E1 oligomerization upon exposure to low pH and target liposomes and showed an enhancement of oligomerization with cholesterol-containing membranes. The E1 homotrimer may represent a perfusion complex that requires cholesterol to carry out the final coalescence of the viral and target membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony R. P., Paredes A. M., Brown D. T. Disulfide bonds are essential for the stability of the Sindbis virus envelope. Virology. 1992 Sep;190(1):330–336. doi: 10.1016/0042-6822(92)91219-k. [DOI] [PubMed] [Google Scholar]
- Bron R., Wahlberg J. M., Garoff H., Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993 Feb;12(2):693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J., Mann E., Brown D. T. Conformational changes in Sindbis virus envelope proteins accompanying exposure to low pH. J Virol. 1983 Mar;45(3):1090–1097. doi: 10.1128/jvi.45.3.1090-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn D. C., Meyer W. J., Mackenzie J. M., Jr, Johnston R. E. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J Virol. 1990 Aug;64(8):3643–3653. doi: 10.1128/jvi.64.8.3643-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Mason D., White J. M. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J Virol. 1990 Oct;64(10):5106–5113. doi: 10.1128/jvi.64.10.5106-5113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kielian M., Wellsteed J., Mellman I., Rudnick G. Effects of monovalent cations on Semliki Forest virus entry into BHK-21 cells. J Biol Chem. 1985 May 10;260(9):5691–5697. [PubMed] [Google Scholar]
- Helenius A., von Bonsdorff C. H. Semlike Forest virus membrane proteins. Preparation and characterization of spike complexes soluble in detergent-free medium. Biochim Biophys Acta. 1976 Jul 15;436(4):895–899. doi: 10.1016/0005-2736(76)90421-1. [DOI] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Kielian M. C., Helenius A. Role of cholesterol in fusion of Semliki Forest virus with membranes. J Virol. 1984 Oct;52(1):281–283. doi: 10.1128/jvi.52.1.281-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Keränen S., Käriäinen L., Helenius A. Membrane fusion mutants of Semliki Forest virus. J Cell Biol. 1984 Jan;98(1):139–145. doi: 10.1083/jcb.98.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Helenius A. pH-induced alterations in the fusogenic spike protein of Semliki Forest virus. J Cell Biol. 1985 Dec;101(6):2284–2291. doi: 10.1083/jcb.101.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Jungerwirth S., Sayad K. U., DeCandido S. Biosynthesis, maturation, and acid activation of the Semliki Forest virus fusion protein. J Virol. 1990 Oct;64(10):4614–4624. doi: 10.1128/jvi.64.10.4614-4624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Levy-Mintz P., Kielian M. Mutagenesis of the putative fusion domain of the Semliki Forest virus spike protein. J Virol. 1991 Aug;65(8):4292–4300. doi: 10.1128/jvi.65.8.4292-4300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Wahlberg J. M., Garoff H. Spike protein oligomerization control of Semliki Forest virus fusion. J Virol. 1990 Oct;64(10):5214–5218. doi: 10.1128/jvi.64.10.5214-5218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkonen A., von Bonsdorff C. H., Renkonen O. Characterization of Semliki Forest virus grown in mosquito cells. Comparison with the virus from hamster cells. Virology. 1977 May 1;78(1):331–335. doi: 10.1016/0042-6822(77)90105-2. [DOI] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Wellsteed J., Kern H., Harms E., Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W. J., Gidwitz S., Ayers V. K., Schoepp R. J., Johnston R. E. Conformational alteration of Sindbis virion glycoproteins induced by heat, reducing agents, or low pH. J Virol. 1992 Jun;66(6):3504–3513. doi: 10.1128/jvi.66.6.3504-3513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar A., Koblet H. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology. 1988 Sep;166(1):17–23. doi: 10.1016/0042-6822(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Omar A., Koblet H. The use of sulfite to study the mechanism of membrane fusion induced by E1 of Semliki Forest virus. Virology. 1989 Jan;168(1):177–179. doi: 10.1016/0042-6822(89)90418-2. [DOI] [PubMed] [Google Scholar]
- Phalen T., Kielian M. Cholesterol is required for infection by Semliki Forest virus. J Cell Biol. 1991 Feb;112(4):615–623. doi: 10.1083/jcb.112.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Wahlberg J. M., Lobigs M., Liljeström P., Garoff H. Membrane fusion process of Semliki Forest virus. II: Cleavage-dependent reorganization of the spike protein complex controls virus entry. J Cell Biol. 1992 Jan;116(2):349–357. doi: 10.1083/jcb.116.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S., Fuchs R., Kielian M., Helenius A., Mellman I. Acidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutants. J Cell Biol. 1989 Apr;108(4):1291–1300. doi: 10.1083/jcb.108.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Adv Protein Chem. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Doms R. W., Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Morselt H. W., Scholma J., Wilschut J. Fusion of influenza virus in an intracellular acidic compartment measured by fluorescence dequenching. Biochim Biophys Acta. 1987 Nov 2;904(1):165–170. doi: 10.1016/0005-2736(87)90100-3. [DOI] [PubMed] [Google Scholar]
- Stegmann T., White J. M., Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990 Dec;9(13):4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. M., Boere W. A., Garoff H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J Virol. 1989 Dec;63(12):4991–4997. doi: 10.1128/jvi.63.12.4991-4997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J. M., Bron R., Wilschut J., Garoff H. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J Virol. 1992 Dec;66(12):7309–7318. doi: 10.1128/jvi.66.12.7309-7318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J. M., Garoff H. Membrane fusion process of Semliki Forest virus. I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992 Jan;116(2):339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderli-Allenspach H., Günthert M., Ott S. Inactivation of PR8 influenza virus through the octadecylrhodamine B chloride membrane marker. Biochemistry. 1993 Jan 26;32(3):900–907. doi: 10.1021/bi00054a022. [DOI] [PubMed] [Google Scholar]



