Abstract
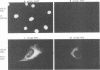
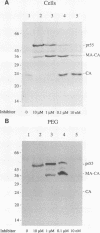
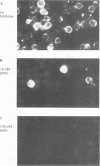
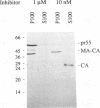
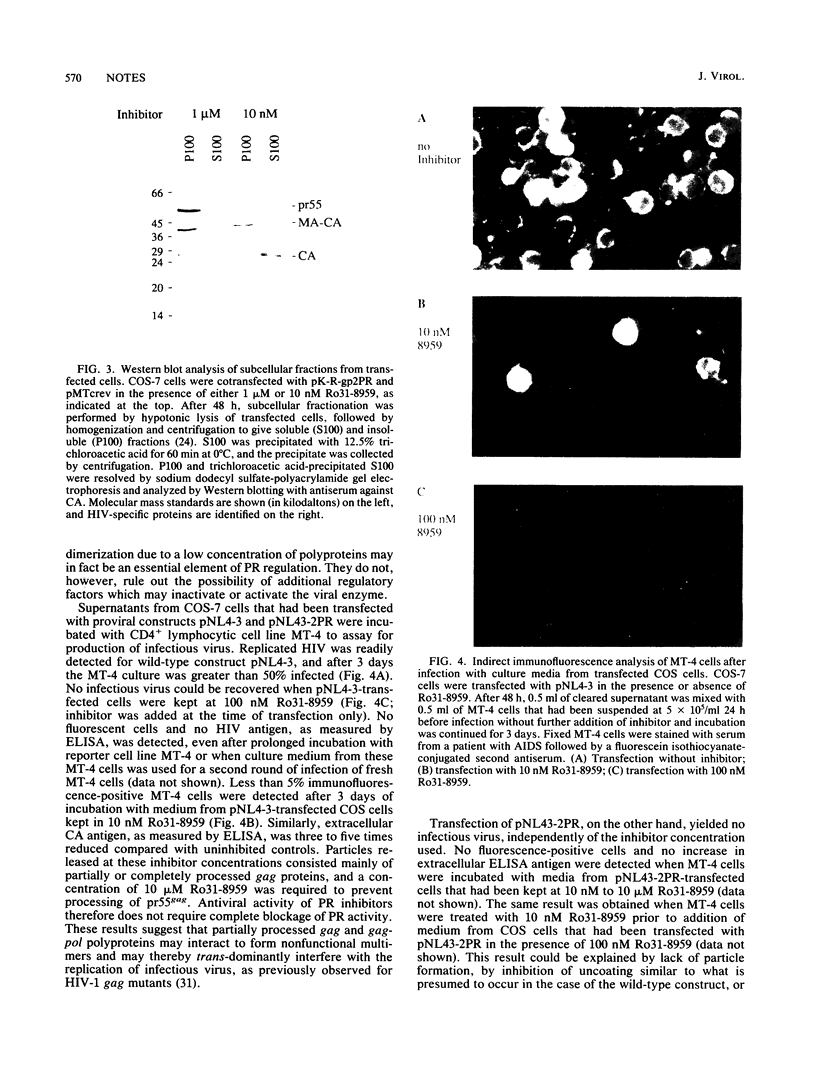
The active form of the retroviral proteinase (PR) is a homodimer of monomeric subunits expressed as integral parts of the viral gag-pol precursor polyproteins, and dimerization of polyproteins is presumed to be important for regulation of PR activity. Expression of a single-chain dimer of the human immunodeficiency virus (HIV) type 1 PR as a component of the viral polyprotein has been shown to prevent particle assembly and viral infectivity (H.-G. Kräusslich, Proc. Natl. Acad. Sci. USA 88:3213-3217, 1991). Ro31-8959, a specific inhibitor of HIV PR, blocked proteolysis of polyproteins containing either wild-type or single-chain dimer PR at the same inhibitor concentration. Different inhibitor concentrations gave three phenotypic effects for the linked PR: at a concentration of 10 nM, cytotoxicity was prevented yet viral polyproteins were almost completely processed and no particles were released. The majority of HIV capsid proteins was found in the soluble cytoplasmic fraction, whereas at a concentration of 1 microM inhibitor most HIV gag proteins were associated with an insoluble fraction. Release of particles consisting of partially processed polyproteins was observed at 100 nM Ro31-8959, and polyprotein processing was blocked at 10 microM. Particles derived from the dimer-containing provirus were noninfectious independently of the inhibitor concentration. Production of infectious HIV after transfection of wild-type provirus was abolished at 100 nM and markedly reduced at 10 nM Ro31-8959.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashorn P., McQuade T. J., Thaisrivongs S., Tomasselli A. G., Tarpley W. G., Moss B. An inhibitor of the protease blocks maturation of human and simian immunodeficiency viruses and spread of infection. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7472–7476. doi: 10.1073/pnas.87.19.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Weber I. T., Cameron C. E., Leis J. P., Skalka A. M. A range of catalytic efficiencies with avian retroviral protease subunits genetically linked to form single polypeptide chains. J Biol Chem. 1991 Mar 15;266(8):4951–4958. [PubMed] [Google Scholar]
- Cheng Y. S., Yin F. H., Foundling S., Blomstrom D., Kettner C. A. Stability and activity of human immunodeficiency virus protease: comparison of the natural dimer with a homologous, single-chain tethered dimer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9660–9664. doi: 10.1073/pnas.87.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiIanni C. L., Davis L. J., Holloway M. K., Herber W. K., Darke P. L., Kohl N. E., Dixon R. A. Characterization of an active single polypeptide form of the human immunodeficiency virus type 1 protease. J Biol Chem. 1990 Oct 5;265(28):17348–17354. [PubMed] [Google Scholar]
- Erickson J., Neidhart D. J., VanDrie J., Kempf D. J., Wang X. C., Norbeck D. W., Plattner J. J., Rittenhouse J. W., Turon M., Wideburg N. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990 Aug 3;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- Hadzopoulou-Cladaras M., Felber B. K., Cladaras C., Athanassopoulos A., Tse A., Pavlakis G. N. The rev (trs/art) protein of human immunodeficiency virus type 1 affects viral mRNA and protein expression via a cis-acting sequence in the env region. J Virol. 1989 Mar;63(3):1265–1274. doi: 10.1128/jvi.63.3.1265-1274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Kräusslich H. G., Wimmer E. Proteolytic processing of polyproteins in the replication of RNA viruses. Biochemistry. 1989 Dec 26;28(26):9881–9890. doi: 10.1021/bi00452a001. [DOI] [PubMed] [Google Scholar]
- Kaplan A. H., Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J., Dunn B. M. Viral proteinases: weakness in strength. Biochim Biophys Acta. 1990 Jan 30;1048(1):1–18. doi: 10.1016/0167-4781(90)90015-t. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G. Human immunodeficiency virus proteinase dimer as component of the viral polyprotein prevents particle assembly and viral infectivity. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3213–3217. doi: 10.1073/pnas.88.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Schneider H., Zybarth G., Carter C. A., Wimmer E. Processing of in vitro-synthesized gag precursor proteins of human immunodeficiency virus (HIV) type 1 by HIV proteinase generated in Escherichia coli. J Virol. 1988 Nov;62(11):4393–4397. doi: 10.1128/jvi.62.11.4393-4397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Lapatto R., Blundell T., Hemmings A., Overington J., Wilderspin A., Wood S., Merson J. R., Whittle P. J., Danley D. E., Geoghegan K. F. X-ray analysis of HIV-1 proteinase at 2.7 A resolution confirms structural homology among retroviral enzymes. Nature. 1989 Nov 16;342(6247):299–302. doi: 10.1038/342299a0. [DOI] [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade T. J., Tomasselli A. G., Liu L., Karacostas V., Moss B., Sawyer T. K., Heinrikson R. L., Tarpley W. G. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990 Jan 26;247(4941):454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- Meek T. D., Dayton B. D., Metcalf B. W., Dreyer G. B., Strickler J. E., Gorniak J. G., Rosenberg M., Moore M. L., Magaard V. W., Debouck C. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1841–1845. doi: 10.1073/pnas.86.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek T. D., Lambert D. M., Dreyer G. B., Carr T. J., Tomaszek T. A., Jr, Moore M. L., Strickler J. E., Debouck C., Hyland L. J., Matthews T. J. Inhibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature. 1990 Jan 4;343(6253):90–92. doi: 10.1038/343090a0. [DOI] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Highly specific antibody to Rous sarcoma virus src gene product recognizes a novel population of pp60v-src and pp60c-src molecules. J Cell Biol. 1985 Feb;100(2):409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière Y., Blank V., Kourilsky P., Israël A. Processing of the precursor of NF-kappa B by the HIV-1 protease during acute infection. Nature. 1991 Apr 18;350(6319):625–626. doi: 10.1038/350625a0. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Shoeman R. L., Höner B., Stoller T. J., Kesselmeier C., Miedel M. C., Traub P., Graves M. C. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6336–6340. doi: 10.1073/pnas.87.16.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeman R. L., Kesselmier C., Mothes E., Höner B., Traub P. Non-viral cellular substrates for human immunodeficiency virus type 1 protease. FEBS Lett. 1991 Jan 28;278(2):199–203. doi: 10.1016/0014-5793(91)80116-k. [DOI] [PubMed] [Google Scholar]
- Tomasselli A. G., Howe W. J., Hui J. O., Sawyer T. K., Reardon I. M., DeCamp D. L., Craik C. S., Heinrikson R. L. Calcium-free calmodulin is a substrate of proteases from human immunodeficiency viruses 1 and 2. Proteins. 1991;10(1):1–9. doi: 10.1002/prot.340100102. [DOI] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Wallin M., Deinum J., Goobar L., Danielson U. H. Proteolytic cleavage of microtubule-associated proteins by retroviral proteinases. J Gen Virol. 1990 Sep;71(Pt 9):1985–1991. doi: 10.1099/0022-1317-71-9-1985. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]