Abstract
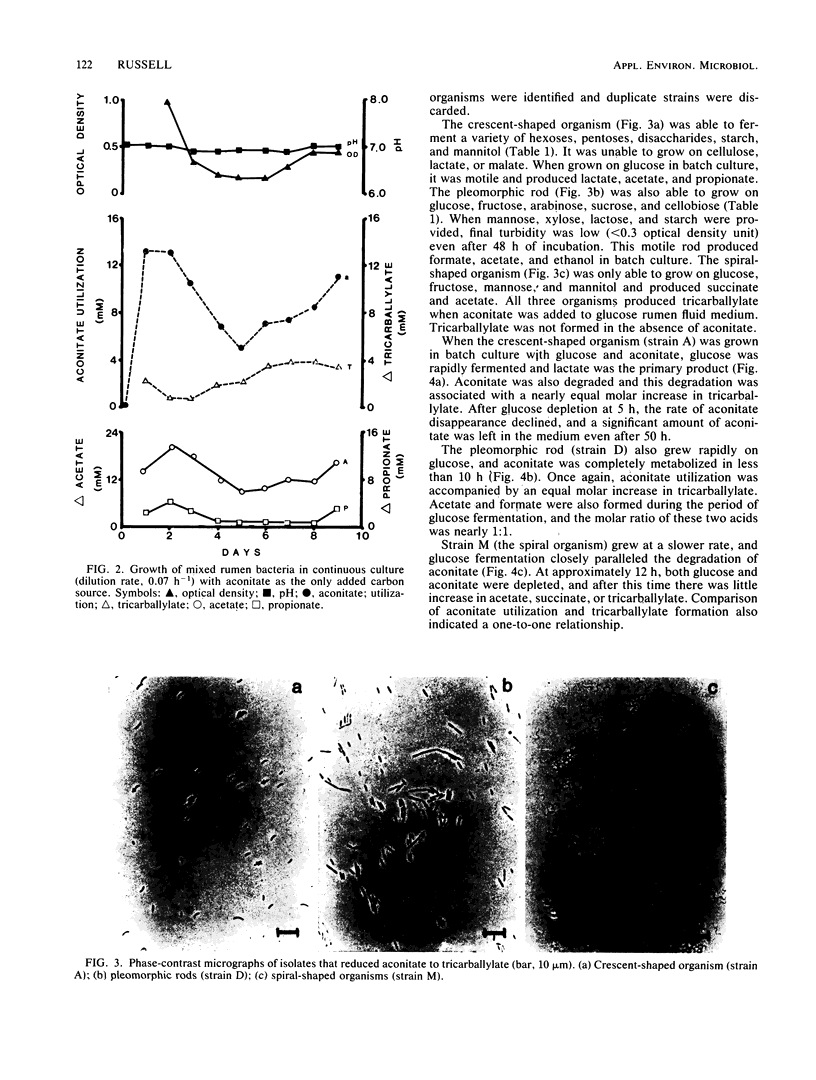
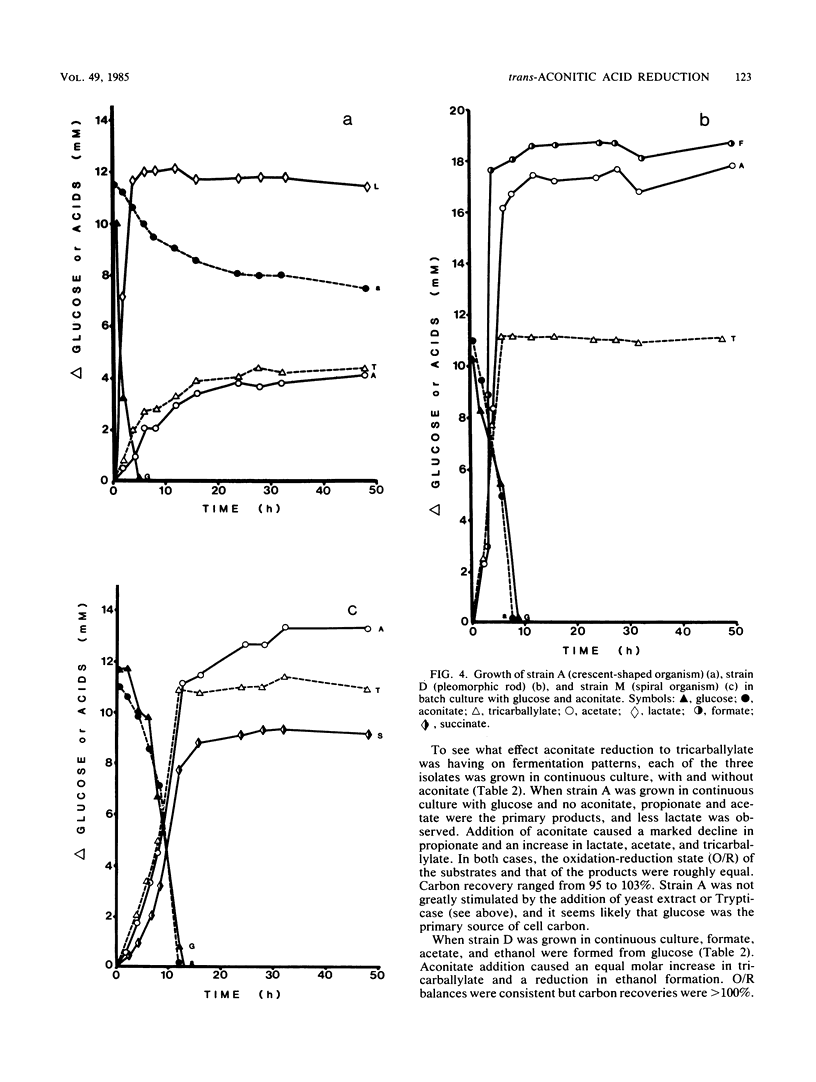
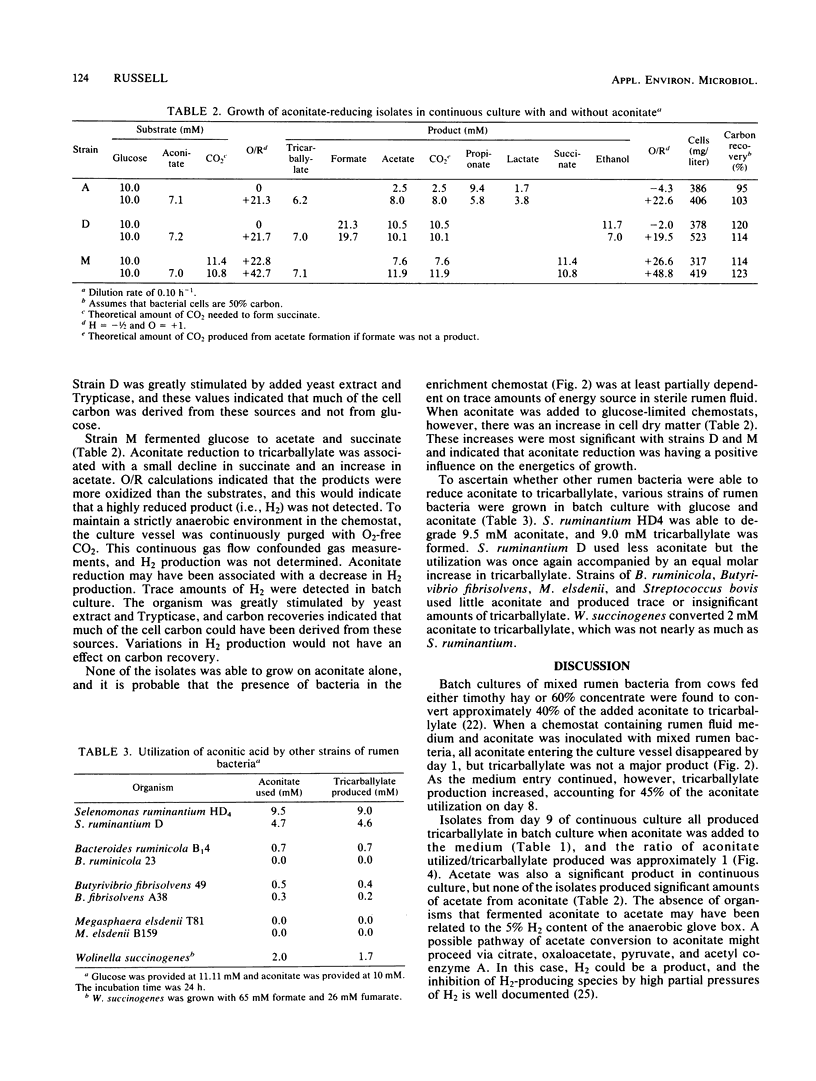
Bacteria from the bovine rumen capable of reducing trans-aconitate to tricarballylate were enriched in an anaerobic chemostat containing rumen fluid medium and aconitate. After 9 days at a dilution rate of 0.07 h−1, the medium was diluted and plated in an anaerobic glove box. Three types of isolates were obtained from the plates (a crescent-shaped organism, a pleomorphic rod, and a spiral-shaped organism), and all three produced tricarballylate in batch cultures that contained glucose and trans-aconitate. In glucose-limited chemostats (0.10 h−1), trans-aconitate reduction was associated with a decrease in the amount of reduced products formed from glucose. The crescent-shaped organism produced less propionate, the pleomorphic rod produced less ethanol, and the spiral made less succinate and possibly H2. Aconitate reduction by the pleomorphic rod and the spiral organism was associated with a significant increase in cellular dry matter. Experiments with stock cultures of predominant rumen bacteria indicated that Selenomonas ruminantium, a species taxonomically similar to the crescent-shaped isolate, was an active reducer of trans-aconitate. Strains of Bacteroides ruminicola, Butyrivibrio fibrisolvens, and Megasphaera elsdenii produced little if any tricarballylate. Wolinella succinogenes produced some tricarballylate. Based on its stability constant for magnesium (Keq = 115), tricarballylate could be a factor in the hypomagnesemia that leads to grass tetany.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., WOOD W. A., EMERY R. S. LACTATE METABOLISM BY PEPTOSTREPTOCOCCUS ELSDENII: EVIDENCE FOR LACTYL COENZYME A DEHYDRASE. Biochim Biophys Acta. 1965 Feb 15;97:202–213. doi: 10.1016/0304-4165(65)90084-x. [DOI] [PubMed] [Google Scholar]
- Bohman V. R., Horn F. P., Stewart B. A., Mathers A. C., Grunes D. L. Wheat pasture poisoning. I. An evaluation of cereal pastures as related to tetany in beef cows. J Anim Sci. 1983 Dec;57(6):1352–1363. doi: 10.2527/jas1983.5761352x. [DOI] [PubMed] [Google Scholar]
- Bohman V. R., Lesperance A. L., Harding G. D., Grunes D. L. Induction of experimental tetany in cattle. J Anim Sci. 1969 Jul;29(1):99–102. doi: 10.2527/jas1969.29199x. [DOI] [PubMed] [Google Scholar]
- Burau R., Stout P. R. Trans-Aconitic Acid in Range Grasses in Early Spring. Science. 1965 Nov 5;150(3697):766–767. doi: 10.1126/science.150.3697.766. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. E., Tove S. B. Identification of an endogenous electron donor for biohydrogenation as alpha-tocopherolquinol. J Biol Chem. 1980 May 25;255(10):4447–4452. [PubMed] [Google Scholar]
- Paynter M. J., Elsden S. R. Mechanism of propionate formation by Selenomonas ruminantium, a rumen micro-organism. J Gen Microbiol. 1970 Apr;61(1):1–7. doi: 10.1099/00221287-61-1-1. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of substrate affinities among several rumen bacteria: a possible determinant of rumen bacterial competition. Appl Environ Microbiol. 1979 Mar;37(3):531–536. doi: 10.1128/aem.37.3.531-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallnöfer P., Baldwin R. L. Pathway of propionate formation in Bacteroides ruminicola. J Bacteriol. 1967 Jan;93(1):504–505. doi: 10.1128/jb.93.1.504-505.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



