Abstract
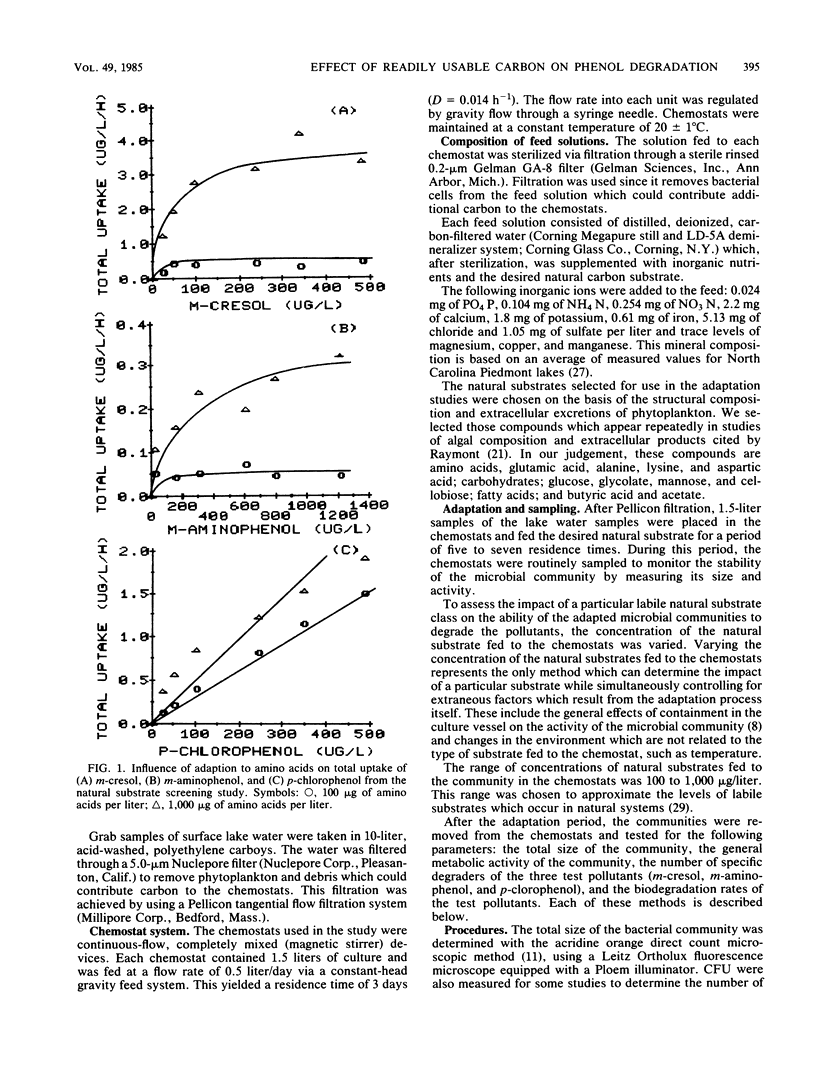
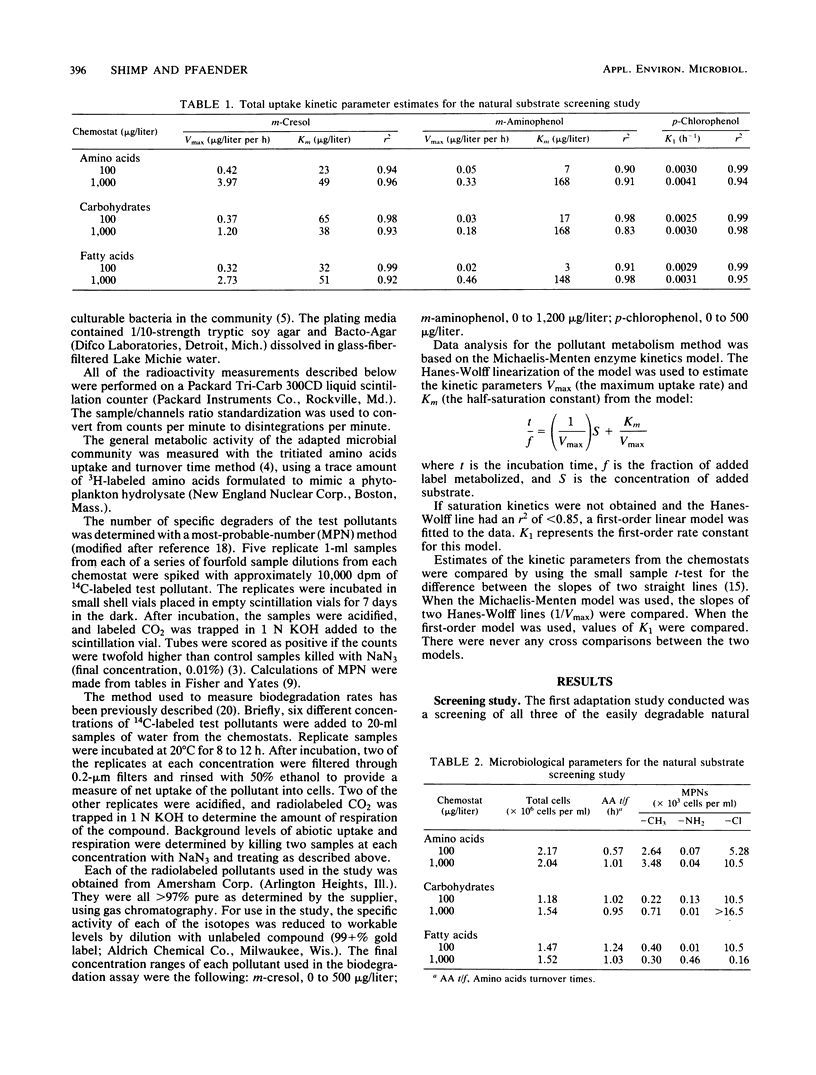
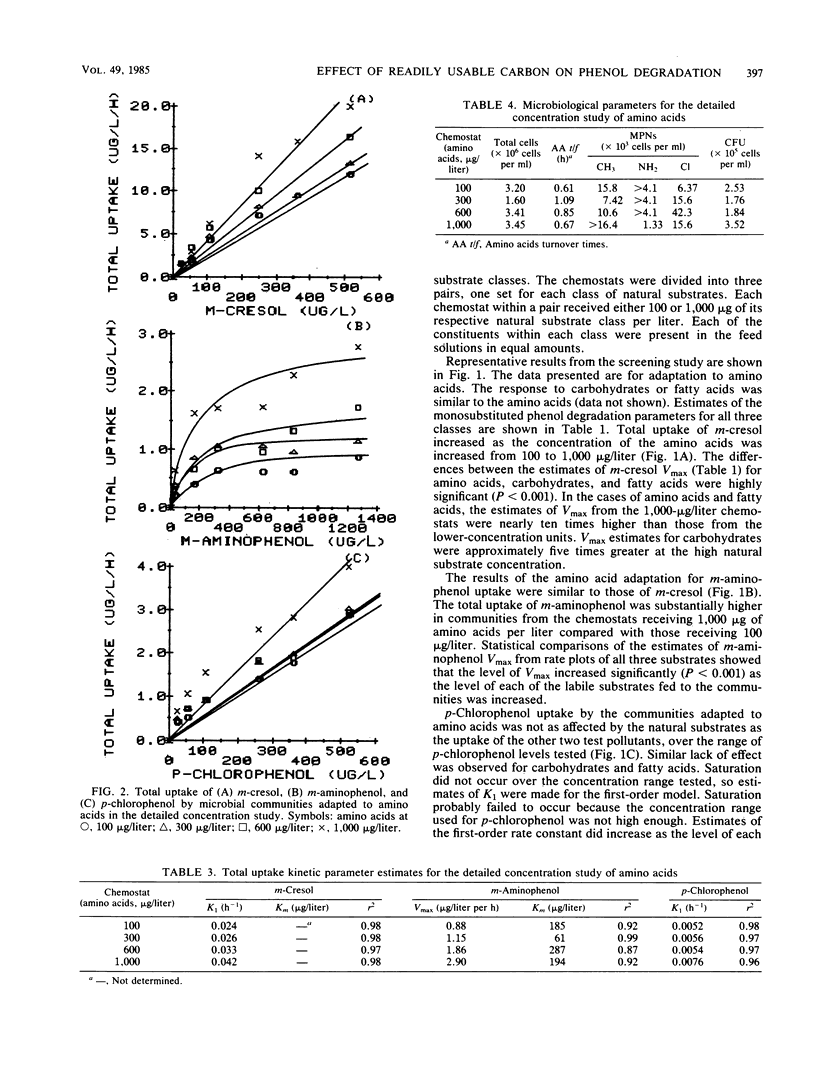
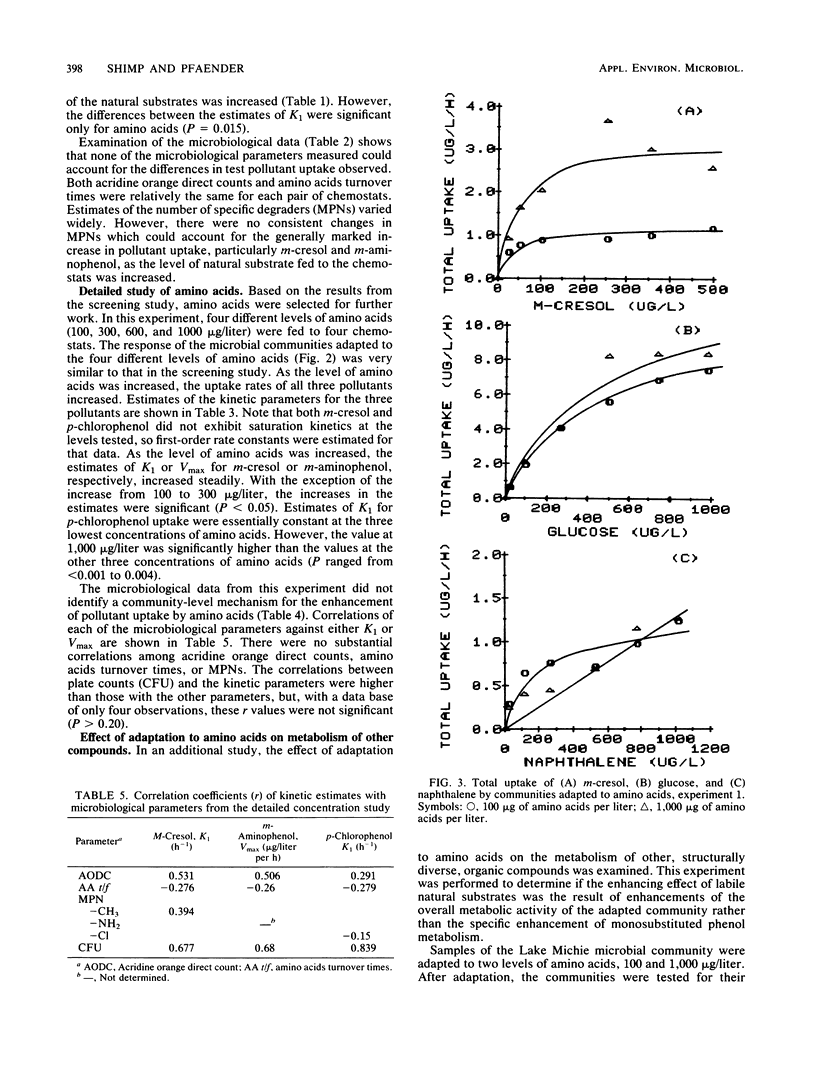
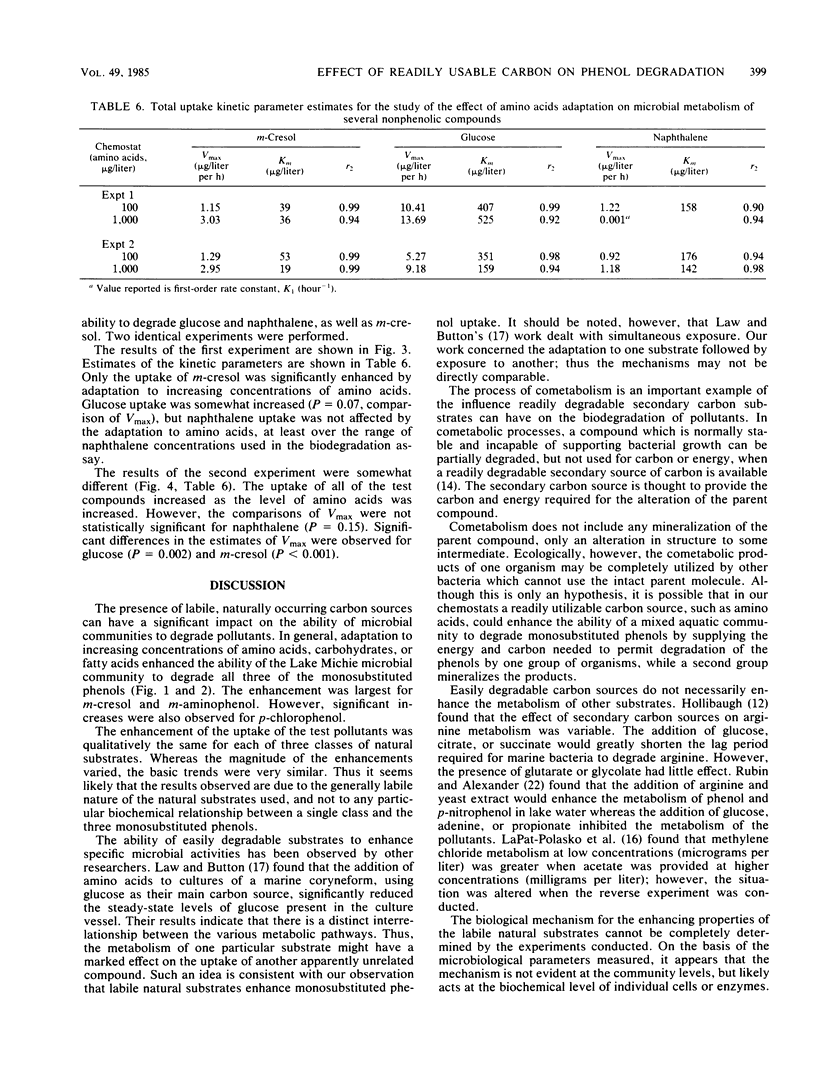
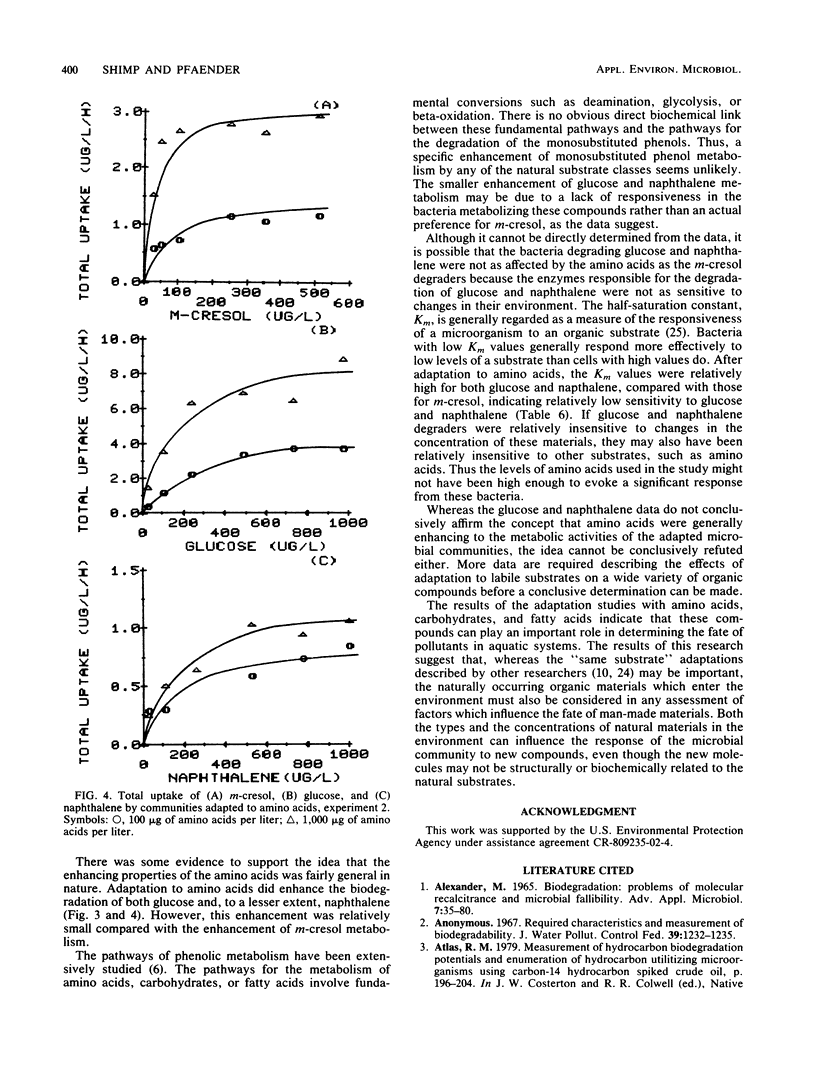
The influence of readily degradable, naturally occurring carbon substrates on the biodegradation of several monosubstitued phenols (m-cresol, m-aminophenol, p-chlorophenol) was examined. The natural substrate classes used were amino acids, carbohydrates, and fatty acids. Samples of the microbial community from Lake Michie, a mesotrophic reservoir, were adapted to different levels of representatives from each natural substrate class in chemostats. After an extended adaptation period, the ability of the microbial community to degrade the monosubstituted phenols was determined by using a radiolabeled substrate uptake and mineralization method. Several microbiological characteristics of the communities were also measured. Adaptation to increasing concentrations of amino acids, carbohydrates, or fatty acids enhanced the ability of the microbial community to degrade all three phenols. The stimulation was largest for m-cresol and m-aminophenol. The mechanism responsible for the enhancement of monosubstituted phenol metabolism was not clearly identified, but the observation that adaptation to amino acids also increased the biodegradation of glucose and, to a lesser extent, naphthalene suggests a general stimulation of microbial metabolism. This study demonstrates that prior exposure to labile, natural substrates can significantly enhance the ability of aquatic microbial communities to respond to xenobiotics.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation: problems of molecular recalcitrance and microbial fallibility. Adv Appl Microbiol. 1965;7:35–80. doi: 10.1016/s0065-2164(08)70383-6. [DOI] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol Rev. 1972 Jun;36(2):146–155. doi: 10.1128/br.36.2.146-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPat-Polasko L. T., McCarty P. L., Zehnder A. J. Secondary substrate utilization of methylene chloride by an isolated strain of Pseudomonas sp. Appl Environ Microbiol. 1984 Apr;47(4):825–830. doi: 10.1128/aem.47.4.825-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmicke L. G., Williams R. T., Crawford R. L. 14C-most-probable-number method for enumeration of active heterotrophic microorganisms in natural waters. Appl Environ Microbiol. 1979 Oct;38(4):644–649. doi: 10.1128/aem.38.4.644-649.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender F. K., Bartholomew G. W. Measurement of aquatic biodegradation rates by determining heterotrophic uptake of radiolabeled pollutants. Appl Environ Microbiol. 1982 Jul;44(1):159–164. doi: 10.1128/aem.44.1.159-164.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel R. G., Rich P. H. Carbon in freshwater systems. Brookhaven Symp Biol. 1973 Aug;(30):241–263. doi: 10.2172/4659070. [DOI] [PubMed] [Google Scholar]


