Abstract
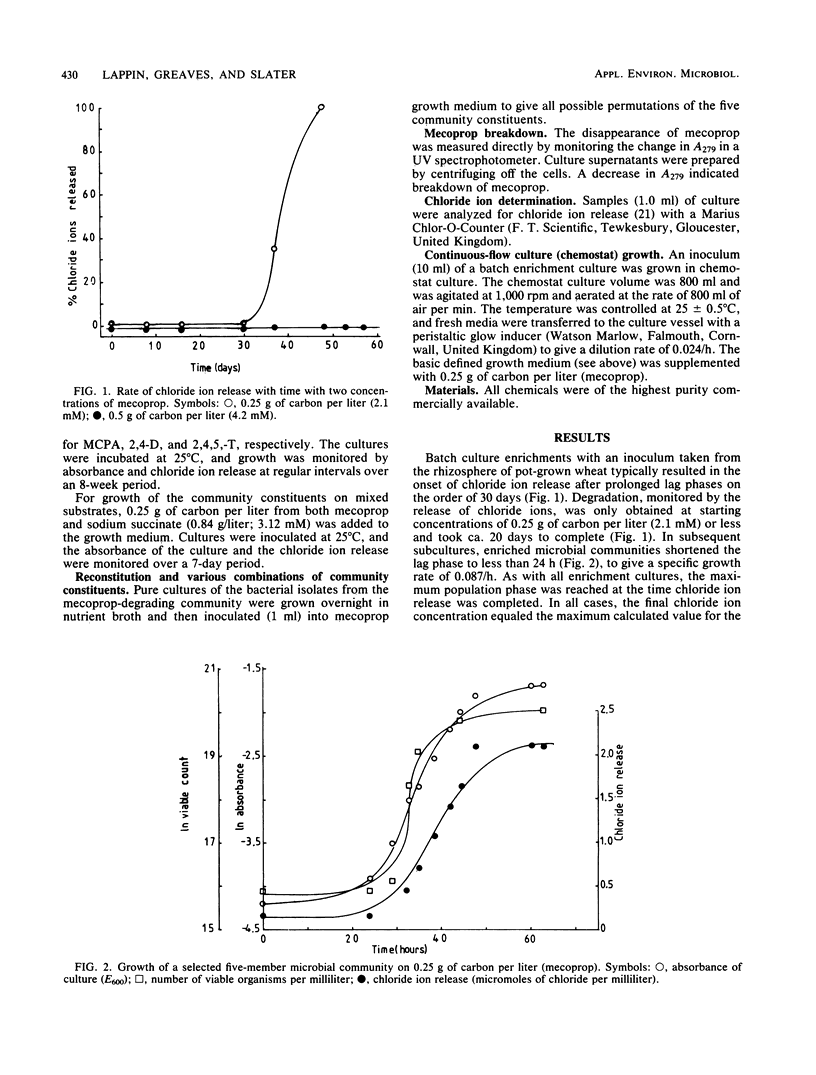
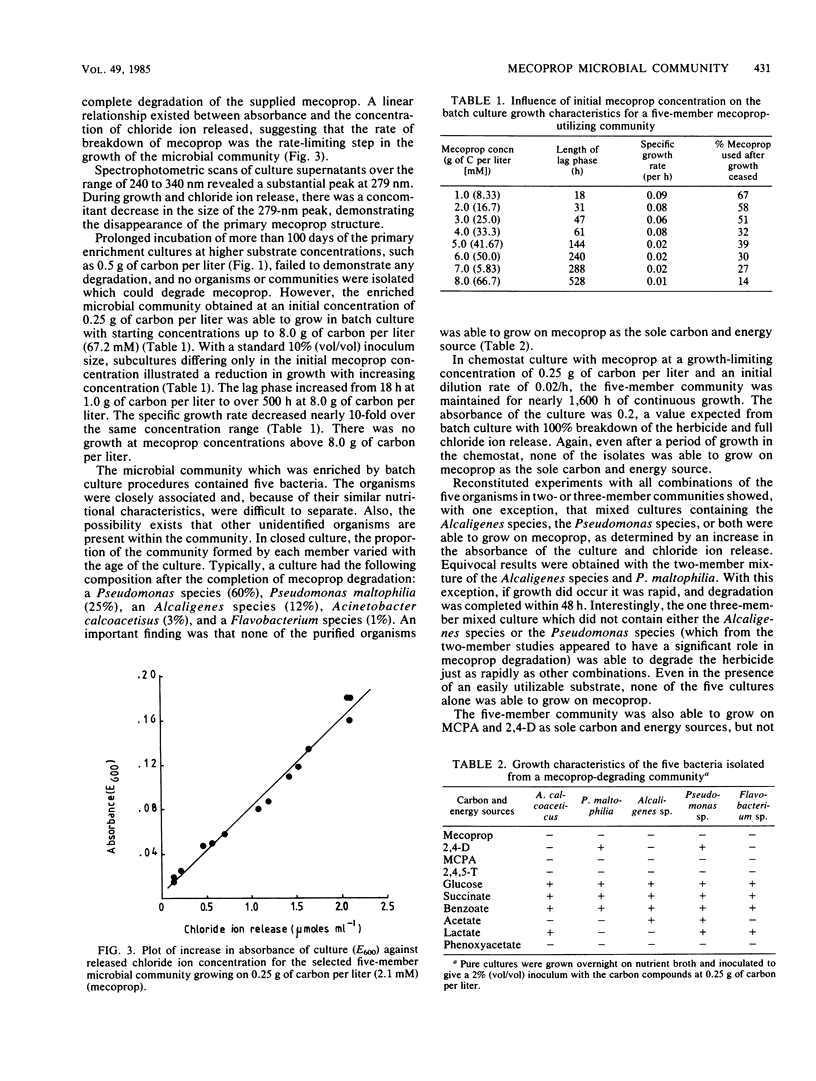
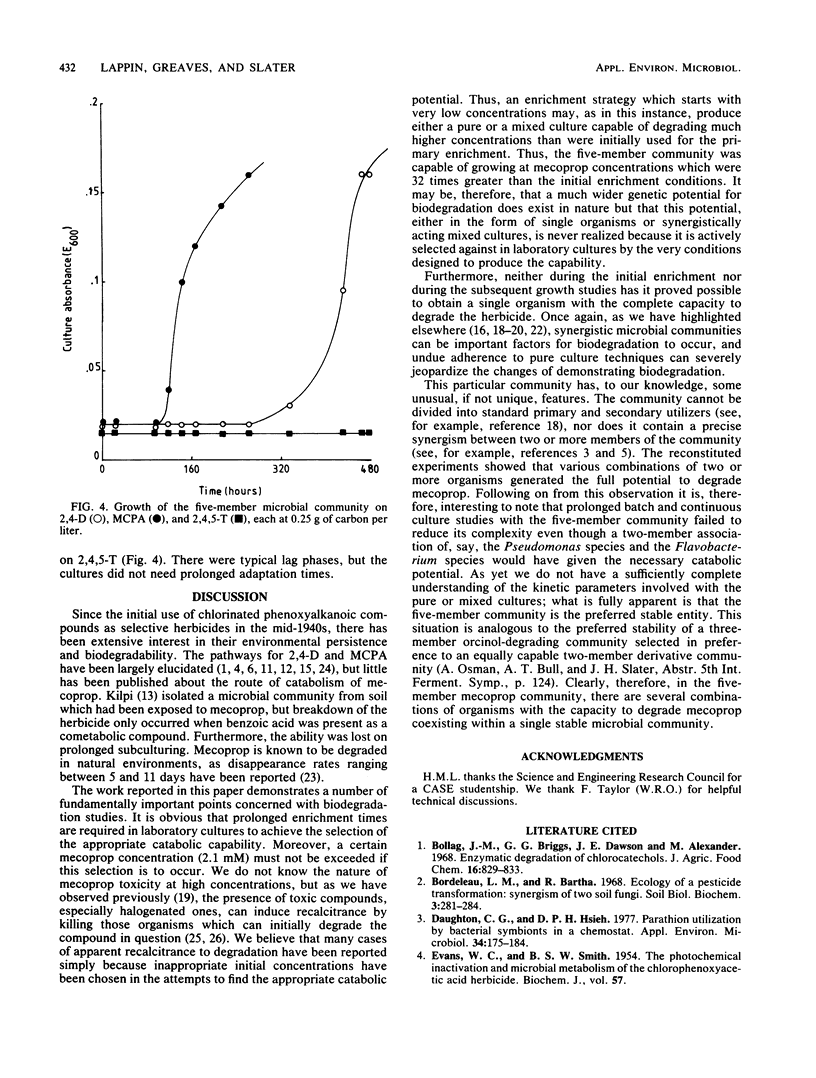
A microbial community isolated from wheat root systems was capable of growth on mecoprop as the sole carbon and energy source. When exposed to fresh herbicide additions, the community was able to shorten the lag phase from 30 days to less than 24 h. The community comprised two Pseudomonas species, an Alcaligenes species, a Flavobacterium species, and Acinetobacter calcoaceticus. None of the pure cultures was capable of growing on mecoprop. Certain combinations of two or more community constituents were required before growth commenced. The mecoprop-degrading community could also degrade 2,4-dichlorophenoxyacetic acid and 2-methyl-4-chlorophenoxyacetic acid but not 2,4,5-trichlorophenoxyacetic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daughton C. G., Hsieh D. P. Parathion utilization by bacterial symbionts in a chemostat. Appl Environ Microbiol. 1977 Aug;34(2):175–184. doi: 10.1128/aem.34.2.175-184.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt J. K., Evans W. C. Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Ring-fission, lactonizing and delactonizing enzymes. Biochem J. 1971 May;122(4):533–542. doi: 10.1042/bj1220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunner H. B., Zuckerman B. M. Degradation of 'Diazinon' by synergistic microbial action. Nature. 1968 Mar 23;217(5134):1183–1184. doi: 10.1038/2171183a0. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Loos M. A., Roberts R. N., Alexander M. Phenols as intermediates in the decomposition of phenoxyacetates by an Arthrobacter species. Can J Microbiol. 1967 Jun;13(6):679–690. doi: 10.1139/m67-090. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Construction of haloaromatics utilising bacteria. Nature. 1979 Feb 1;277(5695):385–386. doi: 10.1038/277385a0. [DOI] [PubMed] [Google Scholar]
- Senior E., Bull A. T., Slater J. H. Enzyme evolution in a microbial community growing on the herbicide Dalapon. Nature. 1976 Oct 7;263(5577):476–479. doi: 10.1038/263476a0. [DOI] [PubMed] [Google Scholar]
- Wigmore G. J., Ribbons D. W. Selective enrichment of Pseudomonas spp. defective in catabolism after exposure to halogenated substrates. J Bacteriol. 1981 Jun;146(3):920–927. doi: 10.1128/jb.146.3.920-927.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore G. J., Ribbons D. W. p-Cymene pathway in Pseudomonas putida: selective enrichment of defective mutants by using halogenated substrate analogs. J Bacteriol. 1980 Aug;143(2):816–824. doi: 10.1128/jb.143.2.816-824.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


