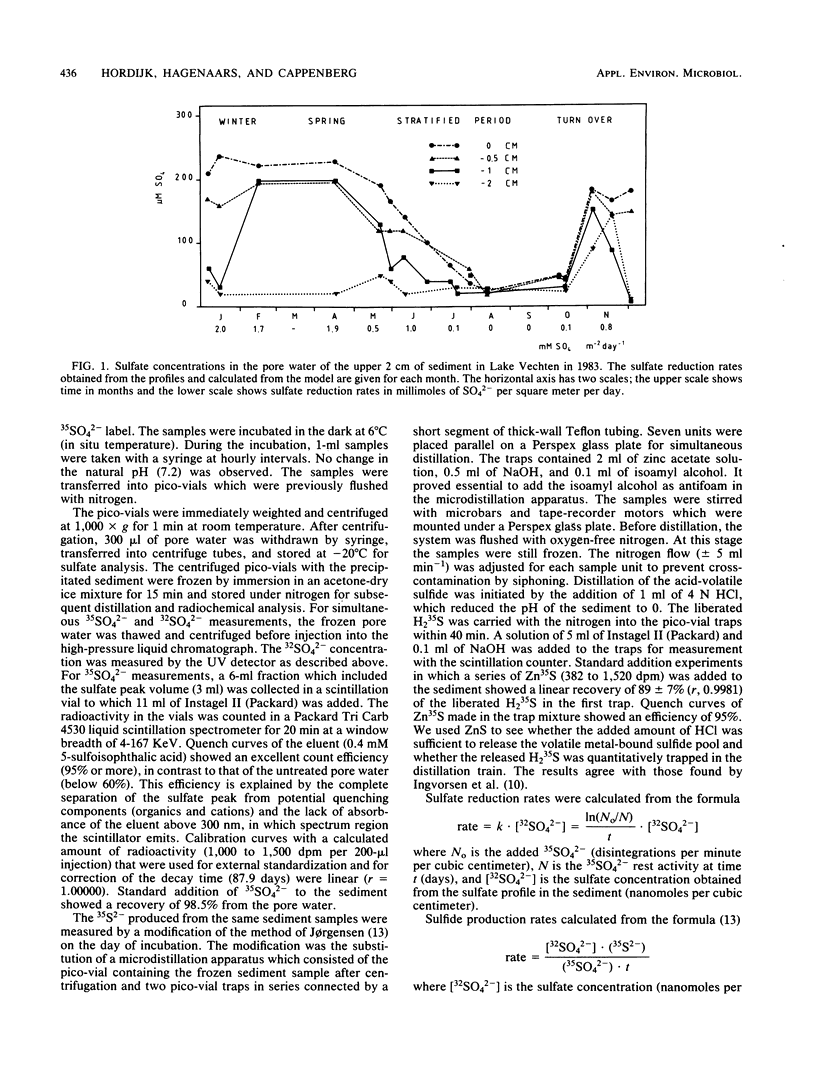
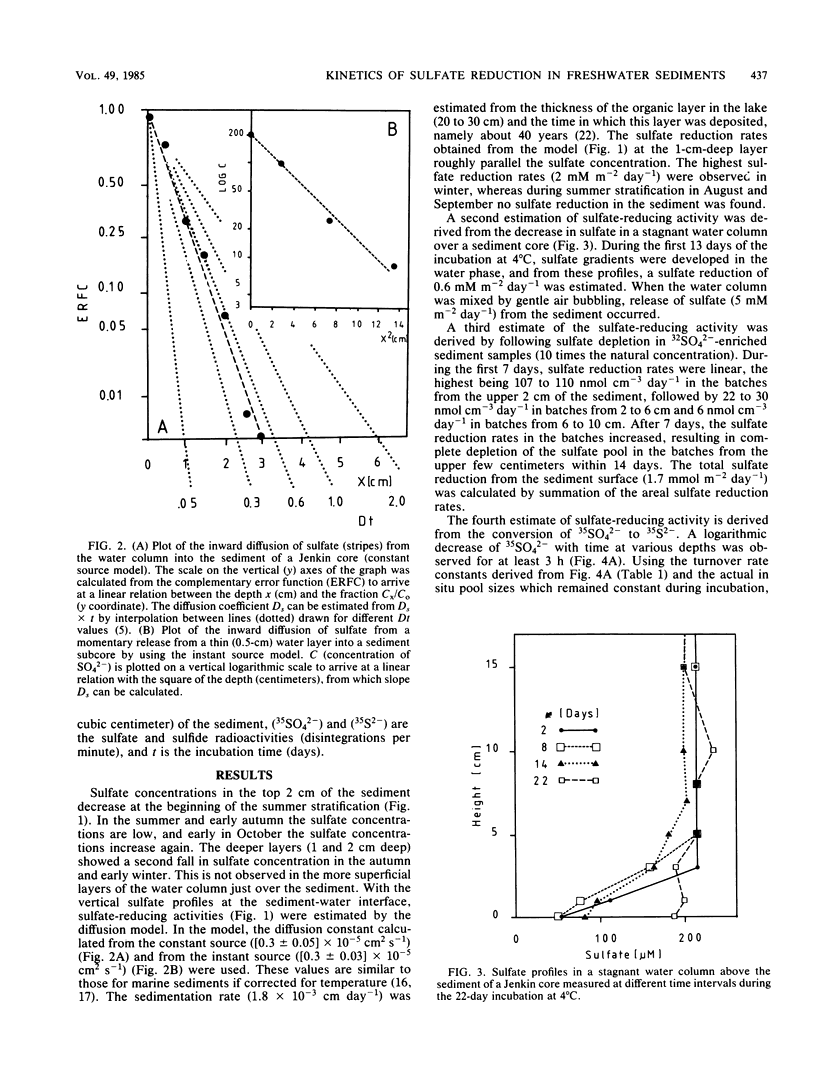
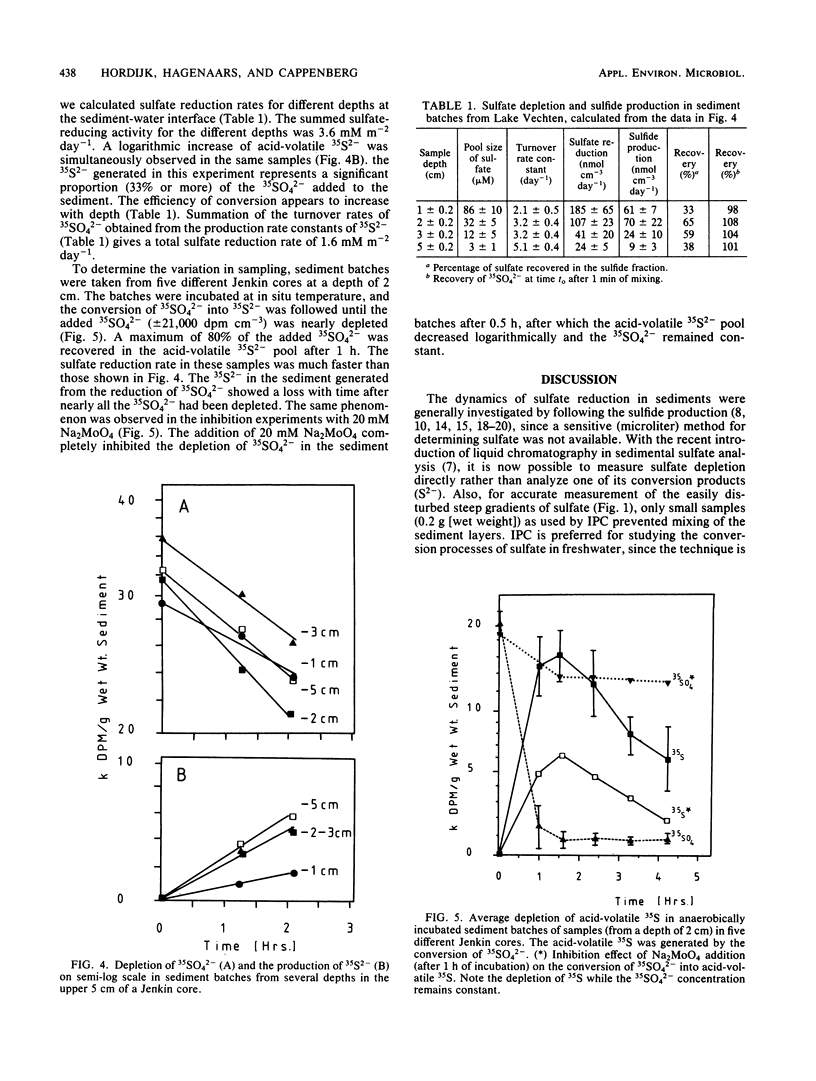
Abstract
Indirect photometric chromatography and microdistillation enabled a simultaneous measurement of sulfate depletion and sulfide production in the top 3 cm of freshwater sediments to be made. The simultaneous measurement of sulfate depletion and sulfide production rates provided added insight into microbial sulfur metabolism. The lower sulfate reduction rates, as derived from the production of acid-volatile 35S2− only, were explained by a conversion of this pool to an undistillable fraction under acidic conditions during incubation. A mathematical model was applied to calculate sulfate reduction from sulfate gradients at the sediment-water interface. To avoid disturbance of these gradients, the sample volume was reduced to 0.2 g (wet weight) of sediment. Sulfate diffusion coefficients in the model were determined (Ds = 0.3 × 10−5 cm2 s−1 at 6°C). The results of the model were compared with those of radioactive sulfate turnover experiments by assessing the actual turnover rate constants (2 to 5 day−1) and pool sizes of sulfate at different sediment depths.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Hordijk K. A., Cappenberg T. E. Quantitative high-pressure liquid chromatography-fluorescence determination of some important lower Fatty acids in lake sediments. Appl Environ Microbiol. 1983 Aug;46(2):361–369. doi: 10.1128/aem.46.2.361-369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth R. W. Pyrite: its rapid formation in a salt marsh and its importance in ecosystem metabolism. Science. 1979 Jan 5;203(4375):49–51. doi: 10.1126/science.203.4375.49. [DOI] [PubMed] [Google Scholar]
- Ingvorsen K., Zeikus J. G., Brock T. D. Dynamics of bacterial sulfate reduction in a eutrophic lake. Appl Environ Microbiol. 1981 Dec;42(6):1029–1036. doi: 10.1128/aem.42.6.1029-1036.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


