Abstract
Object
The purpose of this study was to evaluate the gene transfer capability and tolerability of plasmid DNA/polyethylenimine (PEI) complexes in comparison with adenovirus and naked plasmid DNA in the canine brain.
Methods
Plasmid or adenoviral vectors encoding firefly luciferase were injected directly into the cerebral parenchyma of five adult dogs at varying doses and volumes. Serial physical and neurological examinations, as well as blood and cerebrospinal fluid (CSF) analyses, were conducted before and after the surgery for 3 days. Three days after gene delivery, a luciferase activity assay and immunofluorescence analysis were used to test the brain tissue for gene expression.
Results
Injection into the brain parenchyma resulted in gene transfer throughout the cerebrum with every vector tested. Luciferase expression was highest when adenovirus vectors were used. Injection of plasmid DNA/PEI complexes and naked DNA resulted in similar levels of luciferase expression, which were on average 0.5 to 1.5% of the expression achieved with adenovirus vectors. Immunofluorescent microscopy analysis revealed that plasmid DNA/PEI complexes transduced mainly neurons, whereas adenovirus transduced mainly astrocytes. No significant acute side effects or neurological complications were observed in any of the dogs. Mononuclear cell counts significantly increased in the CSF after adenovirus injection and modestly increased after injection of plasmid DNA/PEI complexes, suggesting that a mild, acute inflammatory response occurred in the central nervous system (CNS).
Conclusions
Compared with rodent models that are limited by very small brains, the dog is an excellent preclinical model in which to assess the distribution and safety of emerging gene transfer technologies. In this study, short-term gene transfer was evaluated as a prelude to long-term expression and safety studies. The authors conclude that the viral and nonviral vectors tested were well tolerated and effective at mediating gene transfer throughout a large portion of the canine brain. The nonviral plasmid vectors were less effective than adenovirus, yet they still achieved appreciable gene expression levels. Due to reduced gene transfer efficiency relative to viral vectors, nonviral vectors may be most useful when the expressed protein is secreted or exerts a bystander effect. Nonviral vectors offer an alternative means to genetically modify cells within the CNS of large mammals.
Keywords: adenovirus, dog, gene therapy, glioma
Gene transfer into the CNS has shown promise as an emerging treatment for neurodegenerative, genetic, and malignant disease.2,3,7,14,18,21,27,40,42,51 Many vectors, including viral vectors, naked DNA, and chemically protected DNA complexes, are being tested for delivery of therapeutic genes into the brain. Such vectors are commonly tested in rodent models that are limited by the small size of their bodies, which does not allow assessment of volumes and doses that could be used in humans. The significantly different size of rodent and human brain may partially account for discrepancies in preclinical efficacy studies2-4,6 and results obtained in human clinical trials.11,21 In addition, because rodent models of malignant and neurodegenerative diseases are often induced artificially, they are therefore limited in their ability to predict human clinical responses.
Dogs, on the other hand, have very large brains compared with rodents. This makes them an attractive model in which to test gene delivery efficiency, evaluate vector and transgene distribution, and optimize treatment protocols prior to clinical implementation in humans. Furthermore, dogs commonly develop malignant brain tumors and neurological disorders that closely resemble the human diseases.9,45,46 Although some CNS gene therapy studies have been performed in dogs,10,12,35 this model is largely underutilized considering that dogs have spontaneous diseases of the CNS that usually go untreated and the animals die or undergo euthanasia.
Viral vectors are effective and widely used gene delivery tools,15,20,22,25,31,32 but there are considerable benefits in developing nonviral vectors for human gene therapy including ease of large-scale manufacturing, elimination of the risk of vector replication and preexisting immunity to vector, and potential safety advantages relative to viral vectors. Nonviral vector–mediated gene transfer has caused tumor regression in human cancer patients (see the review in Ohlfest et al.41), including patients with malignant glioma.50 Nonviral vectors can be delivered as naked plasmid DNA or as plasmid DNA “complexed” with various chemicals that facilitate protection from the endosome and translocation to the nucleus. Polyethylenimine is a synthetic polycation that has been used as a plasmid delivery vehicle for gene transfer into the brains of rodent models1,26,40 but has never been tested in a large animal model.
In this study we sought to determine the gene transfer capability and tolerability of plasmid DNA/PEI complexes compared with naked DNA and adenovirus in the CNS of canines. This investigation represents the first application of DNA/PEI complexes in a large animal brain at doses that might be used in humans.
Materials and Methods
Canine Model
Five healthy adult beagles were purchased from a licensed vendor. All dogs were kept in a clean and well-maintained canine housing facility at the Veterinary Medical Center at the University of Minnesota. Our experiments were conducted according to an approved Institutional Animal Care and Use Committee protocol and every effort was made to minimize pain and discomfort. The housing area was maintained in a 12-hour light/12-hour dark cycle at 22 ± 2°C throughout the study. Every dog was fed ad libitum 220 g of standard maintenance diet daily.
Anesthesia and Surgery/Gene Delivery
Before the surgery, the dogs were premedicated with acepromazine (0.01 mg/kg intramuscularly) for the sedation. An intravenous catheter was placed in a cephalic vein and anesthesia was induced with sodium thiopental (5 mg/kg). A cuffed endotracheal tube was placed and a light plane of anesthesia was maintained using 2% (end-tidal) isoflurane in 100% O2 at a flow rate of 2 L/minute. Lactated Ringers solution (10 ml/kg/hr) was administered intravenously during anesthesia. Carprofen, a nonsteroidal antiinflammatory drug (2.2 mg/kg orally every 12 hours) and buprenorphine (0.01 mg/kg intravenously and subcutaneously every 4–6 hours as needed) were used for postoperative analgesia.
A skin incision was made along the dorsal midline of the skull to expose the sagittal crest. The fasciae of the superficial and deeper temporalis muscles were incised and the temporalis muscles elevated and reflected laterally to expose the skull from the nasion to the inion. Five holes were made in the skull using a 1.5-mm drill bit. The holes were centered between the nasion and inion, approximately 1.5 cm lateral to the sagittal crest and spaced 1 cm apart. Ligatures of 3-0 polyglycolic acid suture (Vicryl) and hemostatic forceps were used for hemostasis. An 18-gauge hypodermic needle was used to make a stab incision in the dura mater, and the tip of the infusion catheter was advanced 2 cm into the cerebral cortex directed in a paramedian plane.
For bolus injections, 8.19 × 108 pfu of the adenovirus was diluted in sterile saline in a final volume of 50 μl, and five bilateral injections were given (spaced 1 cm apart at a depth of 2 cm [from the cortical surface]; 5 μl/site with a total of 10 injections [five injections/cerebral hemisphere]). Bolus injection of plasmid vectors was performed identically to that of the adenovirus, only the volume was increased to 20 μl/site, as described in the Results. Two dogs received one injection into each cerebral hemisphere that was given at a depth of 2 cm (from cortical surface) in a 200-μl volume over 10 minutes using a kdScientific 100 micropump. Bone wax was packed around the catheter in the drill hole to stabilize the catheter during infusion of the vectors. The temporalis fascia was apposed with 3-0 polydioxanone in a simple continuous pattern. The superficial musculature and subcutaneous tissues were closed with 3-0 polyglycolic acid in a simple continuous pattern and the skin edges reapposed with 3-0 Ethilon nylon in interrupted cruciate sutures.
Scale Up of RAdLuc (Firefly Luciferase)
The RAdLuc used for this study is a first-generation replication-defective recombinant adenovirus Type 5 vector expressing firefly luciferase under the transcriptional control of the human cytomegalovirus intermediate early promoter within the E1 region. The RAd-Luc vector seed stock was constructed as described previously.28 The vector was scaled up in our laboratory by infecting the human embryonic kidney HEK 293 cell line with three infectious units/cell of vector seed stock. Cells were harvested 72 hours later and purified by three-step CsCl gradients as previously detailed.43 The vector was titered in triplicate by end-point dilution, cytopathic effect assay. The titer determined was 8.19 × 1010 pfu/ml. The vector preparation was screened for the presence of replication-competent adenovirus16,43 and for lipopolysaccharide contamination (Cambrex).13,43 The virus preparations used were free from replication-competent adenovirus and lipopolysaccharide contamination. The expression of firefly luciferase encoded within the adenovirus was verified by infecting the vector onto COS-7 cells with a multiplicity of infection of 100 pfu/cell and measuring bioluminescence activity 72 hours later using the Promega dual luciferase reporter assay system.
Plasmid Vectors
The plasmid DNA vector used has been described.40 Briefly, the expression of firefly luciferase was regulated by a strong CMV/β–actin chimeric promoter. Plasmid DNA used in this study was prepared using a Qiagen endotoxin-free Maxi prep kit. Linear 22-kD PEI (ExGen 500) was purchased from Fermentas (Lithuania). The DNA/PEI complexes were prepared as previously described.40 Naked DNA was diluted in sterile 5% dextrose immediately prior to gene delivery.
Monitoring and Killing of Dogs
The dogs’ body temperatures were taken with a rectal thermometer. Blood and CSF samples, from the cisternae magnae, were collected immediately before surgery and 3 days thereafter. Complete blood count, serum chemistry analysis, urinalysis, and CSF evaluation were performed by associates in the clinical pathology department in the Veterinary Medical Center at the University of Minnesota. Three days after surgery the dogs were killed by intravenous administration of sodium pentobarbital (200 mg/kg). Thereafter, all dogs were perfused with freshly prepared Tyrode solution (132 mM NaCl/1.8 mM CaCl2 0.32 mM NaH2PO4 5.56 mM glucose/11.6 mM NaHCO3 2.68 mM KCl) with 500 U of heparin with O2 aeration by cardiac perfusion. In the dog brain that was to be used for immunofluorescence, tyrode perfusion was followed by perfusion with 4% paraformaldehyde. The right cerebral hemisphere of the adenovirally treated dog was homogenized after tyrode perfusion, and the left hemisphere was then placed in 4% paraformaldehyde for immunofluorescence.
Luciferase Assays
Each brain was split into two cerebral hemispheres to be assayed separately. The procedure for vector injection and dissection for gene expression analysis is summarized in Fig. 1. The cerebellum and brainstem were discarded. As shown in Fig. 1D, each hemisphere was cut into three arbitrary regions of equal depth (measured by ruler); the dorsal (superior), medial, and ventral (inferior) pieces of brain were then homogenized mechanically in 1× tissue lysis buffer (Promega). Luciferase assays were conducted using dog brain lysates with a Promega luciferase assay kit according to the manufacturer’s instructions. In Fig. 2 lower, the whole-brain luciferase activity is shown; lysates from all three slices of brain tissue were pooled and assayed. The background luciferase activity was determined by assayed brain tissue obtained in a healthy (noninjected) dog.
FIG. 1.
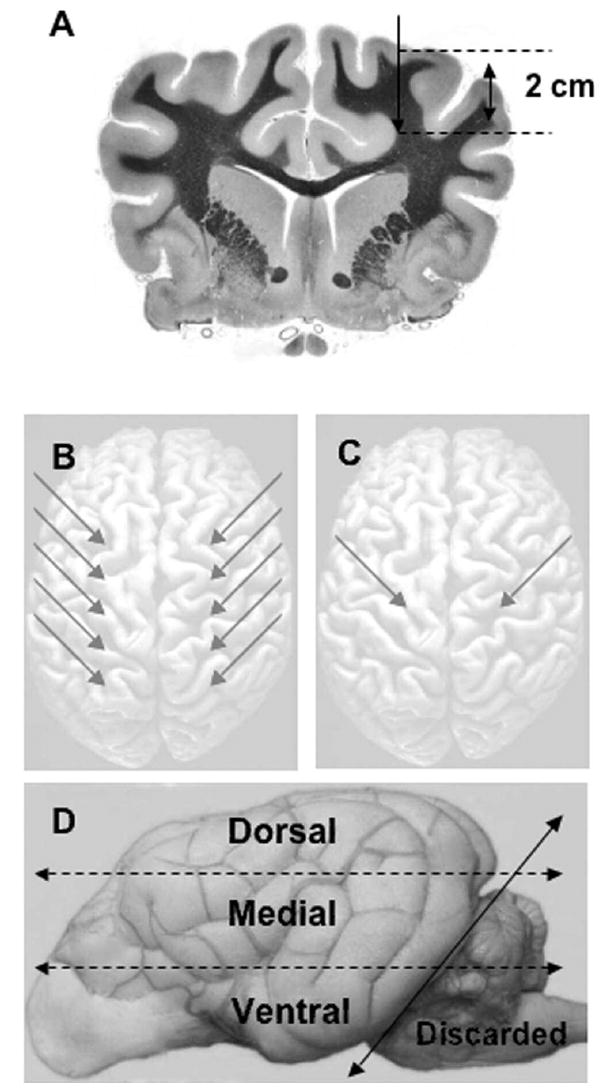
A: Image showing that all plasmid and adenoviral vectors were injected 2 cm beneath the surface of the cortex. B: Schematic showing the bolus injection sites (arrows). One dog was given five bilateral injections of RAdLuc (5 μl/site). Two dogs were given five bilateral injections of plasmid DNA, naked (one animal) or complexed in PEI (one animal) in a volume of 20 μl/site. C: Schematic demonstrating the vector infusion sites (arrows) for slow infusion. Two dogs were given one bilateral injection of DNA/PEI complexes (200 μl/site over 10 minutes). D: Schematic depicting the brain processing for luciferase assays. The left and right cerebral hemispheres were separated. Each hemisphere was sliced into three arbitrary sections (dorsal, medial, and ventral) of equal thickness (dotted lines). Each slice of tissue was then homogenized and assayed for luciferase expression. The cerebellum and brainstem were discarded before assay (solid line).
FIG. 2.
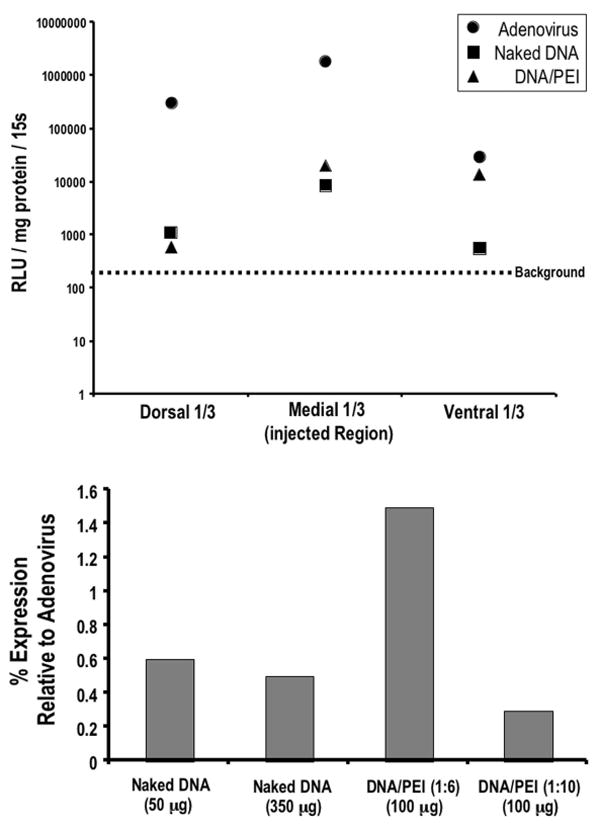
Graphs. Upper: Dogs were bilaterally injected intrace-rebrally with 8.19 × 108 pfu of RAdLuc, plasmid DNA/PEI complexes (50 μg; N/P ratio = 6), or naked DNA (50 μg). One vector-treated cerebral hemisphere obtained in each dog was sliced into three sections (dorsal, medial, and ventral) of equal size, homogenized, and assayed for luciferase activity. Lower: Note the luciferase activity of pooled lysates from the entire cerebral hemisphere (normalized to expression in adenovirally injected brain). RLU = relative light unit; s = second.
Immunofluorescence Evaluation
Thirty-micrometer-thick coronal sections were cut through the striatum by using a microtome. Free-floating immunofluorescence was undertaken to detect firefly luciferase, in combination with neuronal NeuN and glial (GFAP) markers. Sections were blocked with 5% donkey serum (Jackson Immuno Research Laboratories, Inc.) and permeabilized with 0.3% Triton X100 from Fisher Scientific for a 1-hour incubation period before application of the primary antibody. We used rabbit anti-GFAP (Dako Corp.; 1:200 dilution) to detect glial and goat anti–luciferase antibody (Abcam, Inc.; 1:200 dilution) and NeuN antibody to detect mature neuronal cells (Chemicon, Inc.; 1:200 dilution) as primary antibody. The secondary antibody used for fluorescence detection was donkey anti–goat Alexa488 (Molecular Probes; 1:200 dilution) and anti–rabbit Cy5 and anti–mouse Cy3 (Jackson Immuno Research Laboratories, Inc.; 1:200 dilution both). The primary antibodies were incubated overnight at 4°C. The secondary antibody was added the following day for 1 hour at room temperature. All the incubation processes were performed on an orbital shaker. A Bio-Rad MRC 1024 confocal laser head on an upright microscope from Carl Zeiss MicroImaging, Inc., was used for observation of stained sections for immunofluorescence. For observation of H & E–stained slides, we used a Zeiss Atto Arc HBO 110W upright Microscope.
Results
Analysis of Gene Transfer Efficiency and Cell-Type Specific Expression by Luciferase Activity and Immunofluorescence Techniques
Adult beagles received injections of adenoviral or plasmid vectors encoding firefly luciferase into the brain parenchyma under several conditions (injection procedures summarized in Fig. 1). As a positive control for gene transfer and expression, one dog received five bilateral injections of adenovirus into each cerebral hemisphere (5 μl per injection, total of 10 injections). Three days after vector administration, the brain was removed, and the cerebral hemispheres were separated for each half to be analyzed individually. In the adenovirally injected dog, the left hemisphere was immediately fixed for subsequent luciferase immunostaining; the right hemisphere was cut into three equal pieces (a dorsal, medial, and ventral one-third portion), and each section was assayed for gene expression using a luciferase activity assay to determine vector distribution.
The highest luciferase expression was found in the medial portion of the brain where the vector was injected (Fig. 2 upper). However, significant luciferase expression was also found above (dorsal third) and below (ventral third) the injection sites, indicating that adenovirus diffused from the injection site (Fig.2 upper). Accordingly, luciferase immunofluorescence (Fig. 3) revealed that luciferase-positive astrocytes were found mainly near the needle track (Fig. 3B left). Rare luciferase-positive cells were also found in the ventricles, suggesting that some vector diffused into the CSF (Fig. 3B right). To quantify the relative distribution of luciferase-positive cells, the number of positive cells in multiple coronal sections of brain was counted and the distance from the needle track was noted (Fig. 3C). Consistent with the luciferase activity assay (Fig. 2 upper), this histological quantitation revealed that the majority of transduced cells were near the injected region (medial third of the hemisphere).
FIG. 3.
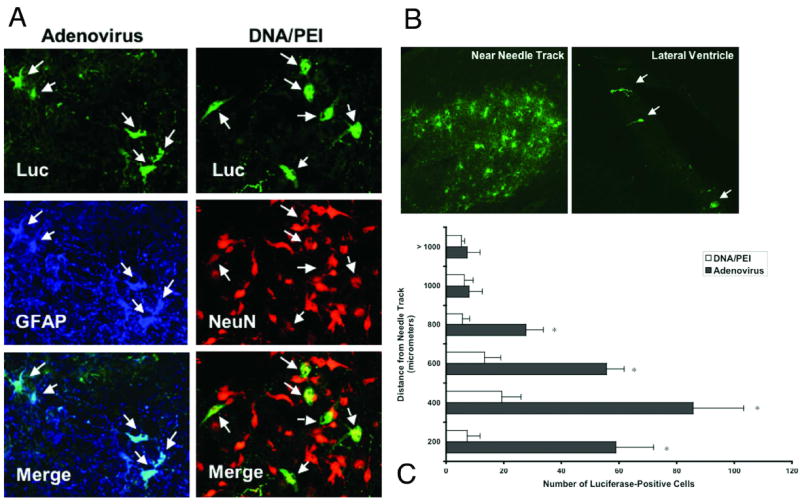
Photomicrographs and bar graph. A: Dogs received a bilateral injection of 100 μg plasmid DNA/PEI complexes or 8.19 × 108 pfu adenovirus encoding luciferase (Luc) into each cerebral hemisphere. Luciferase immunofluorescence staining was conducted along with markers for mature neurons (NeuN) and astrocytes (GFAP). Adenovirus transfected mainly astrocytes (left column), whereas plasmids transfected mainly neurons (right column). Original magnification × 40. B: Representative images of luciferase immunofluorescence. Staining in the adenovirus-treated dog was most intense in areas surrounding the needle track (left). Luciferase-positive cells were also noted several centimeters away from the injection sites lining the ventricles (right). Original magnification × 20. C: Luciferase-positive cells were counted in serial brain sections obtained in dogs treated with adenovirus or DNA/PEI complexes (four sections/dog brain). The distance from the needle track in any direction was noted and the majority of positive cells were within 1 mm of the needle track. There were significantly more luciferase-positive cells near the needle track in the adenovirus-treated dog than the DNA/PEI complex–treated dog. Error bars represent the standard deviations. *p ≤ 0.05 (Student t-test).
The second dog received five bilateral injections of plasmid DNA/PEI complexes such that the left cerebral hemisphere was injected with a total dose of 50 μg of DNA complexed in six equivalents of PEI (N/P ratio = 6; 20 μl per injection, five total injections). The right hemisphere was injected identically, only with 10 Eq PEI (N/P ratio = 10). Three days after vector injection, the brain was removed and both cerebral hemispheres were cut into thirds, homogenized, and assayed for luciferase activity. Similar to the adenovirally injected dog, luciferase expression was detected in all three sections (dorsal–ventral), indicating widespread gene expression from injection into the medial region. The net charge of the DNA/PEI complex was most effective at an N/P ratio of 6 (Fig. 2 lower).
A third dog received five bilateral injections of naked plasmid DNA; the left cerebral hemisphere was injected with a total dose of 50 μg DNA (20 μl per injection, five total injections). The right hemisphere was injected identically with a total dose of 350 μg naked DNA. The brain was processed similarly to the DNA/PEI-injected brain in the aforementioned process; both hemispheres were split into thirds and assayed for luciferase activity. Similar to adenovirus and DNA/PEI complexes, naked DNA gene expression was detected in all three sections of each hemisphere (Fig. 2 upper). The injected hemisphere that received a 50-μg dose had similar luciferase activity to the contralateral hemisphere injected with 350 μg (Fig. 2 lower).
Two other dogs were injected with 100 μg plasmid DNA/PEI complexes in 200 μl over 10 minutes into one site of each cerebral hemisphere (two total injections per dog). The left hemisphere was injected with more neutrally charged complexes (N/P ratio = 6) compared with the right hemisphere (N/P ratio = 10). Three days after vector administration, the brains were fixed and immediately processed for luciferase immunofluorescent analysis. Nearly all luciferase-positive cells colocalized with NeuN, a mature neuronal marker (Fig. 3A). We observed no significant difference in the abundance of luciferase-positive cells between the left and right hemispheres (data not shown). To quantify the distribution of luciferase-positive cells, the cells were counted under a fluorescence microscope and their distance from the needle track was noted. In agreement with the luciferase activity measured on brain lysates, noticeably fewer cells were counted in the dog treated with DNA/PEI complexes. The most luciferase-positive cells were located near the needle track in the adenovirus-treated dogs (Fig. 3B and C). Only rare luciferase-positive cells were seen in the ventricles, indicative of vector diffusion in the CSF.
Side Effects of Gene Delivery
The dogs’ body temperatures were mildly elevated but remained within the normal range after injection in all cases, except in one dog that received a bolus injection of DNA/PEI complexes (Fig. 4). This same dog experienced a mild, acute fever within 12 hours of injection that resolved within 24 hours.8 The CSF analyses showed normal findings, with fewer than 5 WBC/μl in all five dogs prior to gene delivery/surgery. Three days after gene transfer, all dogs that received plasmid DNA had mild to moderate mononuclear pleocytosis (Table 1). The adenovirally treated dog had a marked increase in mononuclear cells in the CSF. This could be due to leakage of the vector into the CSF. The results of H & E staining of brain tissue surrounding the needle track were consistent with the WBC counts in the CSF; adenovirally injected brain exhibited a noticeable increase in mononuclear cell infiltrates surrounding the needle track relative to DNA/PEI injection (Fig. 5).
FIG. 4.
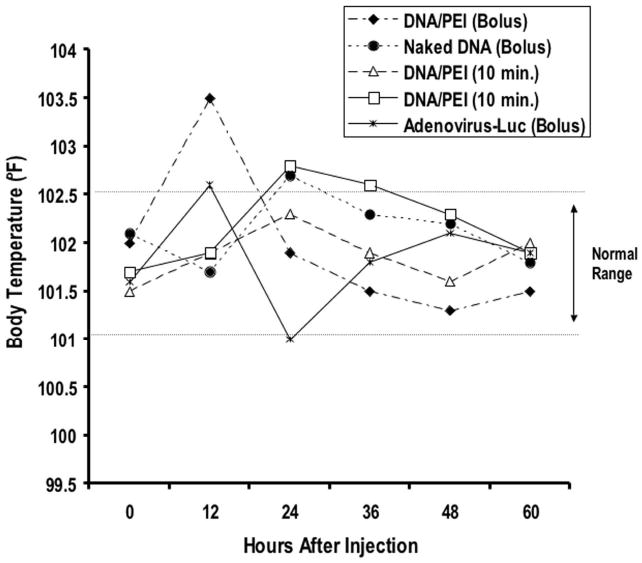
Graph demonstrating rectal body temperatures measured before and every 12 hours after gene delivery.
TABLE I.
Summary of WBC levels measured in the CSF of vector-treated dogs*
| WBC in CSF (μl)
|
||
|---|---|---|
| Vector | Before Injection | 3 Days After Injection |
| adenovirus bolus | 1 | 485 |
| DNA/PEI bolus | 0 | 29 |
| naked DNA bolus | 0 | 7 |
| DNA/PEI (10 mins) | 4 | 45 |
| DNA/PEI (10 mins) | 1 | 25 |
FIG. 5.
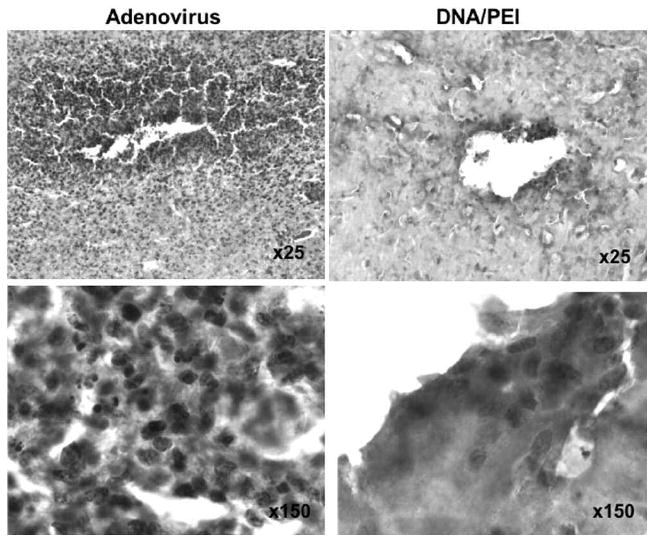
Photomicrographs showing H & E staining of needle track sites in adenovirally and DNA/PEI-injected canine brains. A high number of mononuclear cells were observed infiltrating needle track areas in the adenovirally injected canine brain (left column). The DNA/PEI complex–injected brain showed less infiltration of inflammatory cells (right column). Original magnification appears on the images.
In all dogs we observed mild to moderate mature neutrophilia 24 hours after injection. The WBC counts in the blood returned to the normal range in all dogs that received plasmid DNA. The mild mature neutrophilia persisted 3 days after injection in the dog that had the adenoviral injection. There were no abnormalities in the serum chemical and urine analyses in any dog before or after injection, indicating an absence of acute organ damage (Table 2). In one dog we documented signs of neurological abnormality after gene delivery. The dog that received five bilateral bolus injections (10 total) of DNA/PEI complexes exhibited mild ataxia on recovery from anesthesia; these signs resolved within 24 hours.
TABLE 2.
Results of blood analysis of vector-treated dogs*
| Adenovirus (bolus)
|
DNA/PEI (bolus)
|
Naked DNA (bolus)
|
DNA/PEI (10 mins)
|
DNA/PEI (10 mins)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Ref Range |
Preop | 24 Hrs |
72 Hrs |
Preop | 24 Hrs |
72 Hrs |
Preop | 24 Hrs |
72 Hrs |
Preop | 24 Hrs |
72 Hrs |
Preop | 24 Hrs |
72 Hrs |
| blood urea nitrogen (mg/dl) | 9–31 | 19 | 18 | 18 | 19 | 25 | 15 | 16 | 6 | 12 | 20 | 12 | 10 | 21 | 9 | 10 |
| creatinine (mg/dl) | 0.6–1.6 | 0.9 | 0.7 | 0.7 | 0.6 | 0.9 | 0.7 | 0.6 | 0.4 | 0.6 | 0.7 | 0.5 | 0.7 | 0.7 | 0.5 | 0.7 |
| calcium (mg/dl) | 9.3–11.5 | 10.6 | 10.5 | 10.7 | 10.1 | 10.6 | 10.6 | 10.4 | 10.3 | 10.5 | 10.8 | 10.5 | 11.1 | 10.5 | 10.2 | 10.5 |
| phosphorus (mg/dl) | 3.3–6.8 | 5.3 | 5.7 | 5.9 | 7.4 | 6 | 5.6 | 4.7 | 5.2 | 5.8 | 6.5 | 4.6 | 6 | 6.2 | 5.4 | 6.1 |
| magnesium (mg/dl) | 1.7–2.4 | 1.7 | 1.8 | 1.6 | 1.6 | 1.9 | 1.7 | 1.7 | 1.6 | 1.7 | 1.7 | 1.9 | 1.9 | 1.7 | 1.8 | 1.6 |
| sodium (mmol/L) | 145–153 | 147 | 145 | 146 | 147 | 147 | 146 | 144 | 143 | 144 | 148 | 144 | 144 | 147 | 148 | 146 |
| chloride (mmol/L) | 109–118 | 108 | 111 | 109 | 114 | 114 | 112 | 106 | 111 | 107 | 109 | 105 | 103 | 110 | 112 | 110 |
| potassium (mmol/L) | 3.6–5.3 | 3.4 | 3.9 | 4 | 4.3 | 4.9 | 4.3 | 4.2 | 4.5 | 4.7 | 3.5 | 3.8 | 4.1 | 4.4 | 4.2 | 4.7 |
| bilirubin total | 0–0.3 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| alkaline phosphatase (U/L) | 8–139 | 79 | 81 | 80 | 61 | 100 | 80 | 82 | 162 | 139 | 54 | 138 | 103 | 93 | 167 | 129 |
| gamma glutamyl transferase (U/L) | 0–6 | <3 | <3 | <3 | <3 | 3 | <3 | <3 | 3 | 3 | <3 | <3 | <3 | <3 | 3 | <3 |
| alanine transaminase (U/L) | 22–92 | 57 | 61 | 55 | 30 | 34 | 35 | 30 | 41 | 38 | 55 | 78 | 49 | 30 | 25 | 28 |
| aspartate transaminase (U/L) | 16–44 | 23 | 59 | 15 | 22 | 28 | 20 | 20 | 41 | 19 | 29 | 52 | 15 | 22 | 21 | 17 |
| glucose (mg/dl) | 75–117 | 113 | 108 | 101 | 113 | 94 | 101 | 103 | 111 | 87 | 111 | 115 | 108 | 99 | 111 | 96 |
| cholesterol (mg/dl) | 143–373 | 188 | 180 | 199 | 164 | 196 | 198 | 181 | 181 | 203 | 213 | 256 | 271 | 243 | 245 | 264 |
| amylase (U/L) | 275–1056 | 739 | 657 | 738 | 623 | 575 | 608 | 543 | 415 | 583 | 545 | 380 | 462 | 752 | 467 | 634 |
| WBCs (× 103/μl) | 4.1–13.3 | 8.6 | 11.6 | 12 | 6.6 | 10.6 | 7 | 8.9 | 11.5 | 9.9 | 9.9 | 17.2 | 9 | 10.4 | 15.5 | 9.9 |
| neutrophils, segmented (× 103/μl) | 2.1–11.2 | 5.76 | 9.51 | 9.24 | 3.89 | 8.27 | 3.71 | 3.83 | 8.17 | 6.14 | 6.34 | 15.14 | 6.03 | 7.07 | 13.49 | 6.83 |
| neutrophils, bands (× 103/μl) | 0–0.13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| lymphocytes (× 103/μl) | 0.3–5.1 | 2.06 | 1.39 | 1.56 | 1.65 | 1.7 | 2.45 | 3.56 | 2.19 | 2.38 | 2.87 | 0.69 | 2.16 | 2.39 | 1.24 | 2.48 |
| monocytes (× 103/μl) | 0–1.2 | 0.6 | 0.58 | 0.72 | 0.73 | 0.64 | 0.84 | 1.51 | 1.15 | 1.19 | 0.69 | 1.38 | 0.54 | 0.62 | 0.62 | 0.2 |
| eosinophils (× 103/μl) | 0–1.2 | 0.17 | 0.12 | 0.48 | 0.33 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0 | 0.27 | 0.31 | 0.16 | 0.2 |
| basophils (× 103/ml) | 0–0.13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 |
| hemoglobin (g/dl) | 13.5–19.9 | 15.4 | 12.7 | 13.9 | 14.6 | 14.3 | 13.9 | 15.5 | 16.5 | 17.6 | 14.9 | 15.8 | 16 | 14.2 | 14.1 | 14.2 |
| hematocrit (%) | 38.5–56.7 | 44.3 | 36.4 | 40.2 | 42.6 | 40.3 | 40.6 | 45.2 | 47.9 | 50.5 | 43.2 | 45 | 45.9 | 40.8 | 40.2 | 40.7 |
| platelets (× 103/μl) | 160–425 | 255 | 256 | 259 | ND | 307 | 296 | 228 | 214 | 270 | 283 | 306 | 354 | 378 | 410 | 429 |
ND = no data; Ref= reference.
Discussion
The current study represents the first assessment of the efficacy and tolerance related to the administration of DNA/PEI complexes into a large mammalian brain. Despite the fact the PEI-mediated transfection exhibits toxicity in cultured mammalian cells,5,36 findings in studies in mice1,26,40 and the current study indicate that DNA/PEI complexes are well tolerated when administered into the CNS. One dog in the present study developed acute ataxia after bolus injections of DNA/PEI complexes. Most of the brain tissue in this study was homogenized for luciferase activity assays, which precluded histological analysis of the brain obtained in the ataxic dog. Note that the ataxia resolved within 24 hours. It is possible that any manipulation of the brain, such as simple insertion and removal of 10 needles or catheters may have caused mild signs of neurological deficits. All dogs receiving DNA/PEI complexes had elevated levels of mononuclear cells in the CSF after gene delivery. Injection of the adenovirus caused a more severe pleocytosis compared with naked DNA and DNA/PEI complexes, suggesting that the plasmids may be less inflammatory. We have previously shown in rodent models that adenovirus can cause acute inflammation, which is transient and does not affect the levels or duration of transgene expression.29-31,33,47-49 Alternatively, this could also be due to minor leakage of the adenoviral vectors into the CSF, which has been previously shown to cause inflammation in rodent models.8,44 In addition, it is worth noting that DNA/PEI complexes have been shown not to cause any apparent toxicity after direct administration into the urinary bladders in two patients with refractory bladder cancer.39 The dogs treated with DNA/PEI complexes were followed for 3 days in the present study, which precluded us from collecting data pertaining to long-term safety. Now that we have demonstrated the feasibility of using DNA/PEI complexes to transduce the CNS in the present study, further studies involving long-term end points are justified to establish the long-term impact and duration of transgene expression mediated by DNA/PEI complexes.
The highest levels of gene expression measured by the luciferase activity assay and by immunofluorescence were near the needle track. Peak gene expression was in this medial region where the needle tip was positioned during injection (Figs. 1 and 2), with the majority of luciferase-positive cells near the needle track (Fig. 3B and C). This is not surprising because the injected vector was most likely at high concentration near the needle track. In using the activity assay we also noted luciferase expression in the dorsal and ventral portions of brain tissue (Fig. 2). Scarce luciferase-positive cells were also seen in these regions of brain primarily near the ventricles (Fig. 3B right). We do not expect this was due to cell migration but to diffusion of the vector into the CSF (as evidenced by rare luciferase-positive cells in the ventricles [Fig. 3B right]), retrograde transport of the vector through the CNS, or both. We have previously documented that adenoviral-mediated gene transfer can be detected in the ipsilateral cerebral hemisphere relative to the injection site and that this is likely due to retrograde transport.53 Therefore, in the present study the distribution of transgene expression measured using the luciferase assay is consistent with our histological data and those provided in previous studies.
In the present study we used linear 2-kD PEI and found that in the best-case scenario (N/P ratio = 6) only 1.5% of gene transfer was achieved relative to adenovirus (Fig. 2 lower). Many new formulations of PEI, however, have been developed recently, and these show much greater gene delivery efficiency and reduced toxicity in cultured cells.5,36 In addition, PEI has been conjugated to polyethylene glycol and targeting ligands such as transferrin; in these studies the stability of PEI/polyethylene glycol–transferrin/DNA complexes was increased in the blood,37 and transfection was markedly selective for tumor cells that overexpressed the transferrin receptor.24,38 Kloeckner et al.23 have recently developed EGF/PEI complexes that were up to 30 times more effective relative to unconjugated PEI in cultured cells expressing the EGF receptor. The idea of targeting DNA/PEI complexes to a cell surface receptor is appealing because of the increased efficiency of gene transfer and the ability to target specific cells for gene transfer. Such a strategy could be highly useful in the treatment of gliomas that overexpress tumor-specific receptors such as IL-13α2 and EGF receptor VIII.34,52 Thus, although our results show low levels of gene transfer with a linear 22-kD PEI by local injection, there is little doubt that improved versions of PEI and intravenous delivery would increase the efficacy of this nonviral approach.
High-level gene expression may not always be required if the expressed therapeutic protein is secretable or has a bystander effect. In this case, only transduction of a small number of cells could be sufficient to exert biological activity. For instance, intratumoral injection of naked DNA encoding IL-12 was shown to induce measurable IL-12 in the serum and tumor response in patients with metastatic melanoma.19 Administration of liposomes encoding IL-2 in patients with metastatic renal cell carcinoma demonstrated antitumor activity and safety.17 In both of these clinical studies complete tumor regression was achieved in selected patients.17,19 Furthermore, the authors of the aforementioned clinical trial in which DNA/PEI complexes were used to deliver a suicide gene into bladder carcinoma also demonstrated tumor regression.39 Finally, liposomes encoding a suicide gene have been delivered into malignant glioma and a 50% reduction in tumor volume was observed in two of eight patients.50 These clinical trials have demonstrated that gene transfer involving the use of nonviral vectors is a viable strategy that warrants further investigation. Therefore, even though DNA/PEI complexes were not as effective as adenoviral vectors, this may not prohibit clinical efficacy in the CNS.
Study of the canine models of spontaneous CNS disease will allow future investigators to address delivery, distribution, and safety challenges for gene therapy in the CNS. Unlike rodents, dogs are capable of expressing pain and distress and have brains large enough to allow us to administer doses of vector similar to those that could be used in humans. An example of an underutilized canine model is dogs with spontaneously occurring glioma. Canine glioma shares many features with the human disease such as invasive growth beyond the core tumor mass, similar genetic alterations, peritumoral edema, necrosis, and an extremely dismal prognosis.45 Additional examples of excellent canine models for disease affecting the CNS include those with epilepsy and lysosomal storage disorders. Treatment of spontaneous, rather than artificially induced, CNS disorders in canine models has the potential to accelerate translational gene therapy research and ultimately lead to increased human clinical efficacy.
Conclusions
Plasmid DNA/PEI complexes at an N/P ratio of 6 were the most effective nonviral vectors tested. Naked DNA and more negatively charged (N/P ratio = 10) DNA/PEI complexes also mediated measurable gene transfer. Adenovirus was clearly the most effective gene transfer vector tested but also induced mild local inflammation. Bolus injections and injections given over 10 minutes at multiple locations in the cerebrum were well tolerated with the vectors tested. Long-term efficacy studies will be required to firmly establish the safety of these nonviral vectors. Gene expression was greatest near the injection site but was detected by the luciferase assay throughout the cerebrum, indicating widespread distribution. The dog represents an excellent animal model in which to assess the distribution, efficacy, and safety of gene transfer systems and may be useful in predicting clinical outcomes in human patients.
Acknowledgments
We thank S. Melmed, R. Katzman, and D. Meyer for their academic leadership and superb administrative and organizational support.
We also thank Dr. Harvey Herschman of the University of California, Los Angeles, for his generous gift of the RAdLuc vector seed stock.
The work conducted at the University of Minnesota was supported by grants from the Biomedical Engineering Institute (to J.R.O.). The work performed at the Gene Therapeutics Research Institute was supported by grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) 1R01 NS44556.01, 1 R03 TW006273-01 (to M.G.C.), NIH/NINDS 1 R01 NS 42893.01, U54 NS045309-01, and 1R21 NS047298-01; the Bram and Elaine Goldsmith Chair in Gene Therapeutics (P.R.L.); the Medallion Group Chair in Gene Therapeutics (M.G.C.); the Linda Tallen and David Paul Kane Annual Fellowship; and the Board of Governors at Cedars–Sinai Medical Center.
Abbreviations used in this paper
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EGF
epidermal growth factor
- GFAP
glial fibrillary acidic protein
- IL
interleukin
- PEI
polyethylenimine
- pfu
plaque-forming unit
- WBC
white blood cell
Footnotes
Disclosure The authors declare no conflict of interest with the materials discussed in this paper.
References
- 1.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996;7:1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambar BB, Frei K, Malipiero U, Morelli AE, Castro MG, Lowenstein PR, et al. Treatment of experimental glioma by administration of adenoviral vectors expressing Fas ligand. Hum Gene Ther. 1999;10:1641–1648. doi: 10.1089/10430349950017644. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee P, Weissleder R, Bogdanov A., Jr Linear polyethyleneimine grafted to a hyperbranched poly(ethylene glycol)-like core: a copolymer for gene delivery. Bioconjug Chem. 2006;17:125–131. doi: 10.1021/bc050083e. [DOI] [PubMed] [Google Scholar]
- 6.Biglari A, Bataille D, Naumann U, Weller M, Zirger J, Castro MG, et al. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Ther. 2004;11:721–732. doi: 10.1038/sj.cgt.7700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch A, Perret E, Desmaris N, Heard JM. Long-term and significant correction of brain lesions in adult mucopolysaccharidosis type VII mice using recombinant AAV vectors. Mol Ther. 2000;1:63–70. doi: 10.1006/mthe.1999.0005. [DOI] [PubMed] [Google Scholar]
- 8.Cartmell T, Southgate T, Rees GS, Castro MG, Lowenstein PR, Luheshi GN. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J Neurosci. 1999;19:1517–1523. doi: 10.1523/JNEUROSCI.19-04-01517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandler K. Canine epilepsy: What can we learn from human seizure disorders? Vet J. 2006;172:207–217. doi: 10.1016/j.tvjl.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Chauvet AE, Kesava PP, Goh CS, Badie B. Selective intraarterial gene delivery into a canine meningioma. J Neurosurg. 1998;88:870–873. doi: 10.3171/jns.1998.88.5.0870. [DOI] [PubMed] [Google Scholar]
- 11.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Ciron C, Desmaris N, Colle MA, Raoul S, Joussemet B, Verot L, et al. Gene therapy of the brain in the dog model of Hurler’s syndrome. Ann Neurol. 2006;60:204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- 13.Cotten M, Baker A, Saltik M, Wagner E, Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994;1:239–246. [PubMed] [Google Scholar]
- 14.Cowsill C, Southgate TD, Morrissey G, Dewey RA, Morelli AE, Maleniak TC, et al. Central nervous system toxicity of two adenoviral vectors encoding variants of the herpes simplex virus type 1 thymidine kinase: reduced cytotoxicity of a truncated HSV1-tK. Gene Ther. 2000;7:679–685. doi: 10.1038/sj.gt.3301147. [DOI] [PubMed] [Google Scholar]
- 15.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dion LD, Fang J, Garver RI., Jr Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. J Virol Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- 17.Galanis E, Burch PA, Richardson RL, Lewis B, Pitot HC, Frytak S, et al. Intratumoral administration of a 1, 2-dimyristyloxypropyl-3–dimethylhydroxyethyl ammonium bromide/dioleoylphos-phatidylethanolamine formulation of the human interleukin-2 gene in the treatment of metastatic renal cell carcinoma. Cancer. 2004;101:2557–2566. doi: 10.1002/cncr.20653. [DOI] [PubMed] [Google Scholar]
- 18.Griffey MA, Wozniak D, Wong M, Bible E, Johnson K, Rothman SM, et al. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13:538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Heinzerling L, Burg G, Dummer R, Maier T, Oberholzer PA, Schultz J, et al. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther. 2005;16:35–48. doi: 10.1089/hum.2005.16.35. [DOI] [PubMed] [Google Scholar]
- 20.Hsich G, Sena-Esteves M, Breakefield XO. Critical issues in gene therapy for neurologic disease. Hum Gene Ther. 2002;13:579–604. doi: 10.1089/10430340252837198. [DOI] [PubMed] [Google Scholar]
- 21.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 23.Kloeckner J, Prasmickaite L, Hogset A, Berg K, Wagner E. Photochemically enhanced gene delivery of EGF receptor-targeted DNA polyplexes. J Drug Target. 2004;12:205–213. doi: 10.1080/10611860410001723090. [DOI] [PubMed] [Google Scholar]
- 24.Kursa M, Walker GF, Roessler V, Ogris M, Roedl W, Kircheis R, et al. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug Chem. 2003;14:222–231. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- 25.Lam P, Hui KM, Wang Y, Allen PD, Louis DN, Yuan CJ, et al. Dynamics of transgene expression in human glioblastoma cells mediated by herpes simplex virus/adeno-associated virus amplicon vectors. Hum Gene Ther. 2002;13:2147–2159. doi: 10.1089/104303402320987842. [DOI] [PubMed] [Google Scholar]
- 26.Lemkine GF, Mantero S, Migne C, Raji A, Goula D, Normandie P, et al. Preferential transfection of adult mouse neural stem cells and their immediate progeny in vivo with polyethylenimine. Mol Cell Neurosci. 2002;19:165–174. doi: 10.1006/mcne.2001.1084. [DOI] [PubMed] [Google Scholar]
- 27.Leone P, Janson CG, Bilaniuk L, Wang Z, Sorgi F, Huang L, et al. Aspartoacylase gene transfer to the mammalian central nervous system with therapeutic implications for Canavan disease. Ann Neurol. 2000;48:27–38. doi: 10.1002/1531-8249(200007)48:1<27::aid-ana6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Liang Q, Dmitriev I, Kashentseva E, Curiel DT, Herschman HR. Noninvasive of adenovirus tumor retargeting in living subjects by a soluble adenovirus receptor-epidermal growth factor (sCAREGF) fusion protein. Mol Imaging Biol. 2004;6:385–394. doi: 10.1016/j.mibio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 30.Lowenstein PR. Virology and immunology of gene therapy, or virology and immunology of high MOI infection with defective viruses. Gene Ther. 2003;10:933–934. doi: 10.1038/sj.gt.3302073. [DOI] [PubMed] [Google Scholar]
- 31.Lowenstein PR, Castro MG. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10:946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- 32.Lowenstein PR, Castro MG. Progress and challenges in viral vector-mediated gene transfer to the brain. Curr Opin Mol Ther. 2002;4:359–371. [PubMed] [Google Scholar]
- 33.Lowenstein PR, Castro MG. Recent advances in the pharmacology of neurological gene therapy. Curr Opin Pharmacol. 2004;4:91–97. doi: 10.1016/j.coph.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4:388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhonen MG, Ooboshi H, Welsh MJ, Davidson BL, Heistad DD. Gene transfer to cerebral blood vessels after subarachnoid hemorrhage. Stroke. 1997;28:822–829. doi: 10.1161/01.str.28.4.822. [DOI] [PubMed] [Google Scholar]
- 36.Nimesh S, Goyal A, Pawar V, Jayaraman S, Kumar P, Chandra R, et al. Polyethylenimine nanoparticles as efficient transfecting agents for mammalian cells. J Control Release. 2006;110:457–468. doi: 10.1016/j.jconrel.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 38.Ogris M, Wagner E. Tumor-targeted gene transfer with DNA polyplexes. Somat Cell Mol Genet. 2002;27:85–95. doi: 10.1023/a:1022988008131. [DOI] [PubMed] [Google Scholar]
- 39.Ohana P, Gofrit O, Ayesh S, Al-Sharef W, Mizrahi A, Briman T, et al. Regulatory sequences of the H19 gene in DNA based therapy of bladder cancer. Gene Ther Mol Biol. 2004;8:181–192. [Google Scholar]
- 40.Ohlfest JR, Demorest ZL, Motooka Y, Vengco I, Oh S, Chen E, et al. Combinatorial antiangiogenic gene therapy by nonviral gene transfer using the sleeping beauty transposon causes tumor regression and improves survival in mice bearing intracranial human glioblastoma. Mol Ther. 2005;12:778–788. doi: 10.1016/j.ymthe.2005.07.689. [DOI] [PubMed] [Google Scholar]
- 41.Ohlfest JR, Freese AB, Largaespada DA. Nonviral vectors for cancer gene therapy: prospects for integrating vectors and combination therapies. Curr Gene Ther. 2005;5:629–641. doi: 10.2174/156652305774964749. [DOI] [PubMed] [Google Scholar]
- 42.Passini MA, Macauley SL, Huff MR, Taksir TV, Bu J, Wu IH, et al. AAV vector-mediated correction of brain pathology in a mouse model of Niemann-Pick A disease. Mol Ther. 2005;11:754–762. doi: 10.1016/j.ymthe.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Southgate T, Kingston P, Castro MG. Gene transfer into neural cells in vivo using adenoviral vectors. In: Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. Hoboken, NJ: John Wiley & Sons; 2000. pp. 4.23.21–24.23.40. [Google Scholar]
- 44.Stevenson PG, Hawke S, Sloan DJ, Bangham CR. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71:145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoica G, Kim HT, Hall DG, Coates JR. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41:10–19. doi: 10.1354/vp.41-1-10. [DOI] [PubMed] [Google Scholar]
- 46.Stonehewer J, Mackin AJ, Tasker S, Simpson JW, Mayhew IG. Idiopathic phenobarbital-responsive hypersialosis in the dog: an unusual form of limbic epilepsy? J Small Anim Pract. 2000;41:416–421. doi: 10.1111/j.1748-5827.2000.tb03236.x. [DOI] [PubMed] [Google Scholar]
- 47.Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 48.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci U S A. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 50.Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 51.Watson G, Bastacky J, Belichenko P, Buddhikot M, Jungles S, Vellard M, et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther. 2006;13:917–925. doi: 10.1038/sj.gt.3302735. [DOI] [PubMed] [Google Scholar]
- 52.Wu AH, Xiao J, Anker L, Hall WA, Gregerson DS, Cavenee WK, et al. Identification of EGFRvIII-derived CTL epitopes restricted by HLA A0201 for dendritic cell based immunotherapy of gliomas. J Neurooncol. 2006;76:23–30. doi: 10.1007/s11060-005-3280-7. [DOI] [PubMed] [Google Scholar]
- 53.Zermansky AJ, Bolognani F, Stone D, Cowsill CM, Morrissey G, Castro MG, et al. Towards global and long-term neurological gene therapy: unexpected transgene dependent, high-level, and widespread distribution of HSV-1 thymidine kinase throughout the CNS. Mol Ther. 2001;4:490–498. doi: 10.1006/mthe.2001.0479. [DOI] [PubMed] [Google Scholar]


