Abstract
Since the development of the Edmonton Islet Transplantation Protocol, islet transplantation has given new hope to patients with type 1 diabetes. Like solid organ transplantation, one of the biggest challenges islet transplantation faces is the shortage of available donor tissue. Finding an alternative source of islet tissue has become an increasingly important field of study. The possibility of generating insulin-secreting cells and islet tissue with adult pancreatic stem or progenitor cells has been investigated extensively. This review aims to discuss recent progress in the study of adult pancreatic stem or progenitor cells and to suggest future directions in this field.
Abstract
Depuis l'élaboration du Protocole d'Edmonton sur la transplantation d'îlets, cette forme de transplantation donne un nouvel espoir aux patients atteints de diabète type 1. Comme dans le cas de la transplantation d'organes solides, un des grands défis que doit relever la transplantation d'îlets, c'est la pénurie de tissus de donneurs. La recherche d'une autre source de tissus d'îlets est un domaine d'étude qui prend de l'ampleur. On a beaucoup étudié la possibilité de générer des cellules sécrétrices d'insuline et des tissus d'îlet au moyen de cellules souches pancréatiques ou de cellules progénitrices adultes. Cette étude vise à discuter des progrès réalisés récemment dans l'étude des cellules souches pancréatiques et des cellules progénitrices adultes et suggère de futures orientations dans ce domaine.
Type 1 diabetes (juvenile onset) is a disease caused by the autoimmune destruction of patients' insulin-producing beta cells. The resulting lack of insulin leads to hyperglycemia and its devastating complications, including cardiovascular disease, blindness and kidney failure. People with type 1 diabetes are usually treated with daily insulin injections accompanied by constant monitoring of blood glucose levels.1 Even with meticulous glucose control, such therapy carries the risk of hypoglycemic episodes that can lead to anxiety, seizures, coma and even death.2 Extensive research has been dedicated to discovering new ways to prevent or treat type 1 diabetes and eliminate the need for insulin injections.
Islet transplantation is an alternative, albeit experimental, treatment for patients with type 1 diabetes. Patients receive pancreatic islet cells from cadaveric donors, enabling more precise control of blood glucose levels, compared with traditional insulin therapy. This treatment is also sometimes used in patients with pancreatic trauma, cancer or chronic pancreatitis who require removal of the exocrine pancreas.3–5 In these cases, the pancreatic islets are isolated and returned to the patients by autotransplantation. Islet transplantation, originally proposed by Lacy in 1982,6,7 was limited for many years by its low success rate. In 2000, the development of the Edmonton Islet Transplantation Protocol markedly increased the likelihood of successful islet transplantation due to improved islet isolation techniques and new immunosuppressive regimens.8 Nevertheless, islet transplantation still faces several important challenges, including the need for lifelong immunosuppression and uncertainty regarding the long-term survival and function of human islet grafts. One of the major challenges is the shortage of donor tissue. It has been estimated that less than 1% of all patients with type 1 diabetes could receive islet transplants each year, owing to the critical shortage of tissue.9 This shortage limits the practice of large-scale islet transplantation to all patients with diabetes. Hence, finding an alternative source of islet tissue that can be used in clinical transplantation has become an important field of study in the last few years.
The clinical potential of using stem cells to repair or to replace damaged tissue in diseases such as diabetes has become of increasing interest. In general, a stem cell is an unspecialized cell that can self-renew continuously and differentiate into specific specialized cell types. There are 2 types of stem cells: embryonic stem cells (ES cells) and adult stem cells (Table 1). Pluripotent ES cells maintain the plasticity to generate all types of cells in an individual, except extraembryonic tissue, such as placenta. It was not until recently that several studies demonstrated that adult multipotent stem cells exist, maintain plasticity and may have the capacity to differentiate into several different cell types. For example, adult bone marrow stem cells have been found to give rise to hepatocytes, cardiomyocytes, neural cells and muscle cells; and neural stem cells were shown to generate myocytes and hematopoietic cells.10,11 These adult stem cells may have potential in the treatment of diseases associated with tissue damage and deterioration, such as neurodegenerative disorders12 and heart failure.13 Adult progenitor cells with the ability to regenerate tissue during normal turnover or after damage are of considerable interest, even though their abilities to self-renew and to generate various cell types are greatly restricted14 (Fig. 1).
Table 1
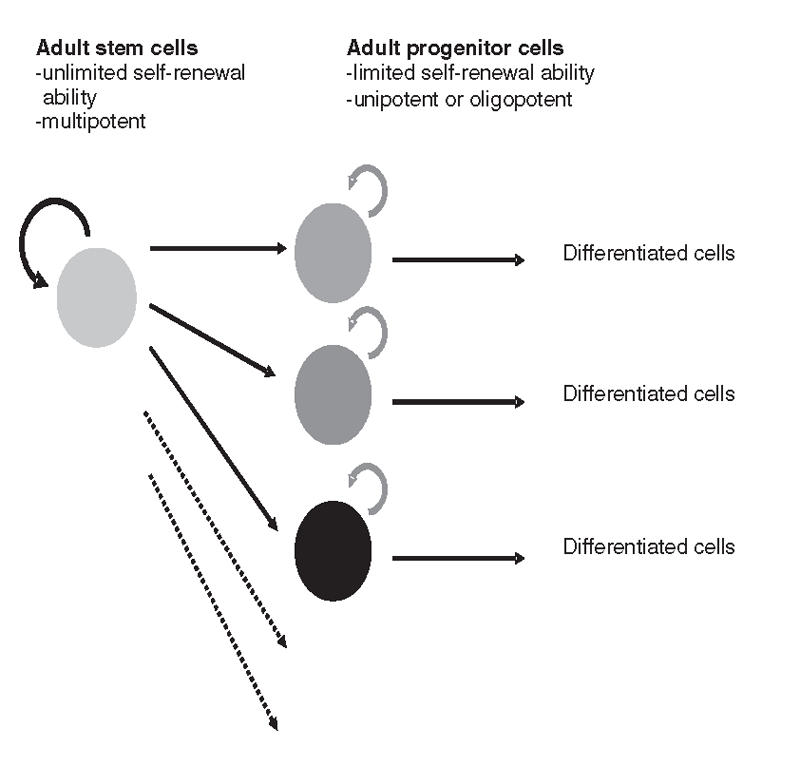
FIG. 1. Renewal ability and potential of adult stem cells and progenitor cells. Adult stem cells maintain the ability to self-renew and to generate various progeny cell types. Adult progenitor cells are limited in their self-renewal ability and in the number of different types of progenies they can generate.
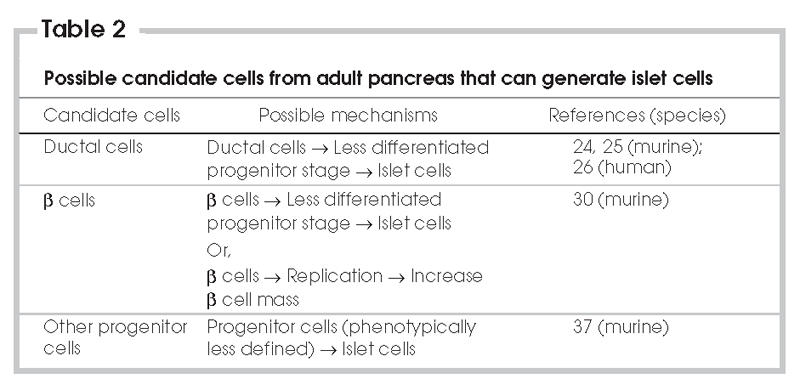
Different cell types have been studied for their potential capacity to differentiate into cells secreting islet hormones, including ES cells, bone marrow stem cells and hepatic stem cells.15,16 Because the adult pancreas has been shown to have self-recovery ability in response to various stresses,17 it has been postulated that stem cells or progenitor cells may exist in the adult pancreas (Table 2). Identifying and isolating these stem cells or progenitor cells would open the door for in vitro generation of islet tissue for use in transplantation. Through a better understanding of the progenitor cell type, islet transplantation protocols might be improved by providing optimal conditions for islet regeneration or by preventing progenitor cell death. Moreover, these stem cells or progenitor cells might be further manipulated through genetic means to prevent immune rejection by the organ recipient. However, it has recently been suggested that beta cell replication, rather than progenitor cell differentiation, may be the primary mechanism responsible for islet regeneration, at least in adult rodents. At the same time, it has been demonstrated that an islet progenitor cell type exists in adult murine pancreas. This review discusses recent progress in the search for adult stem cells and progenitor cells in the pancreas. It will also suggest some directions for future research to discover an alternative source of islet tissue.
Table 2
The pancreatic ductal cell: a possible pancreatic progenitor?
An association between beta cells and pancreatic ducts has been observed in human fetal pancreas, adult rat pancreas and adult human pancreas.18–21 Through histological observation, Jonsson and others found that almost all ductal cells express Pdx-1 (Ipf-1),22 a transcription factor critical in pancreatic development, particularly in islet neogenesis.23 Therefore, it has been speculated that ductal cells are a primary source of new islet cells during islet neogenesis. Cornelius and colleagues first showed that islet-like clusters could be obtained by long-term culture of ductal tissue from adult murine pancreas.24 Remarkably, it was further demonstrated that the implantation of these islet-like clusters could ameliorate diabetes in nonobese mice.24,25 Bonner-Weir and colleagues later confirmed that insulin-producing human islet-like clusters (cultivated human islet buds, or CHIBs) could be generated from human ductal tissue.26 The generation of islet-like clusters with similar or replicated protocols has been reported by other groups using murine or human ductal tissue.27,28 The Bonner-Weir group later suggested that ductal cells possibly move into a less differentiated stage to express Pdx-1, and this allows ductal cells to serve as progenitor cells in the adult pancreas.29 This gives hope for an alternative source of islet tissue, even though current techniques still do not allow the generation of a large number of islet-like clusters, and the clusters express relatively low levels of insulin, compared with pancreatic islets.28 Such studies aimed to show that human ductal cells have the capacity to transdifferentiate into islet cells. The major criticism of these studies is that the starting culture was already contaminated by other pancreatic cell types. The ductal tissue was generally isolated by density gradient centrifugation and handpicking in some cases. Thus, the starting material used by these groups was not likely pure ductal cells, which means contribution of beta cells to the formation of islet-like clusters in these studies cannot be ruled out. Other possibilities, including the involvement of islet cells or multipotent stem or progenitor cells other than ductal or beta cells, might explain these findings. Nevertheless, these studies point to ductal cells as a possible source of new islet cells and demonstrate the feasibility of using ductal tissue to do large-scale expansion and generation of islet-like clusters for future clinical use.
Lineage tracing: the origin of new islet cells
To determine the in vivo origin of new beta cells, Dor and others used a novel genetic lineage tracing method, which allows the identification of beta cells generated from preexisting beta cells and those that have arisen from other sources.30 Insulin expressing, preexisting beta cells are first marked by the expression of human alkaline phosphatase protein (HPAP), using a tamoxifen-inducible Cre/lox system. If new beta cells are generated from cell types that do not express insulin, then as the preexisting HPAP-expressing beta cells start to perish, new, unmarked beta cells will take over the islet. Conversely, if new beta cells are generated from preexisting beta cells, the percentage of HPAP-marked beta cells should remain the same. Using this unique approach, the investigators observed that, after partial pancreatectomy, new beta cells arose in the adult mouse pancreas primarily through beta cell replication and not beta cell neogenesis. This finding has far-reaching implications, because beta cells in adults were considered to be in a fully differentiated stage without significant capacity for proliferation. Moreover, even if adult beta cells can be induced to proliferate and expand, they tend to lose their differentiated status and ability to secrete insulin in vitro.31 The author suggested that the reasons many other findings demonstrate the role of pancreatic stem or progenitor cells in beta cell regeneration are as follows: 1) the progenitor population is insignificantly small and thus makes a negligible contribution to the new beta cell population; 2) the insulin gene is already transcribed in progenitor cells, thus the progenitors in this study would be marked as beta cells; 3) stem cells might act as a source of new beta cells in pathophysiological conditions other than pancreatectomy, such as autoimmune diabetes; and 4) beta cells might be the source of new beta cells, but they need to dedifferentiate transiently into a progenitor state before becoming new beta cells. This study nonetheless points to a significant role of beta cell replication in maintaining beta cell mass in adults. In support of these data, a recent study from Georgia and Bhushan found an inability to maintain beta cell mass in adult mice lacking cyclin D2, a gene critical for beta cell replication.32 This additional evidence further suggests the importance of beta cell replication in generating new beta cells in adults.
There remain several important concerns regarding the study by Dor and colleagues. First, although it was argued that this study demonstrates beta cell replication as the major mechanism to replenish beta cells in the adult pancreas, it does not conclusively prove that beta cell neogenesis from other sources does not occur in the adult pancreas.29 Other points worthy of consideration include the efficiency of the Cre/lox system, sample size used, accuracy of the marking method and time when pancreas samples were collected.29,33 It is conceivable that tamoxifen used to induce Cre gene expression in this system might inhibit beta cell neogenesis.7 Finally, it also remains questionable whether the same beta cell regenerating mechanism occurs during long-term damage or in true type 1 diabetes, as in the pancreatectomy model, and whether this phenomenon exists in humans, as it appears to in rodents. Meier and colleagues' histological study of human type 1 diabetic pancreas from autopsy specimens suggests that extensive beta cell neogenesis must be continuously occurring in the human type 1 diabetic pancreas, because even in patients with long-standing disease, extensive beta cell apoptosis is still occurring.34 Further studies are required to consider the provocative finding by Dor and colleagues and to understand the importance of replication versus neogenesis in the maintenance of beta cell mass.
Identification of pancreatic progenitor cells
Numerous reports have suggested the existence of islet cell progenitors in the adult pancreas. For example, Petropavlovskaia and others suggested that the phenotypically undefined “small cells” are candidates for pancreatic progenitor cells,35 and Suzuki and others proposed that c Met+ cells can differentiate into pancreatic lineages.36 However, until recently, no group had identified and characterized such a progenitor. Seaberg and others used clonal dilution of pancreatic cells obtained from the ductal and islet tissue of mice to isolate cells, termed pancreas-derived multipotent precursors (PMPs), which are capable of differentiating into different pancreas cell types37 (Fig. 2). PMPs are unique in that they express the pancreas precursor marker Pdx-1 and several neural precursor markers. When PMPs were stimulated to differentiate, neural-like cells were generated. At the same time, the PMPs that generated the neural-like cells also had the capacity to give rise to islet-like cells with beta cell, alpha cell or theta cell properties in the same clonal colonies. The percentage of PMP colony-forming cells is only about 0.02%–0.03%. This exciting finding raises the possibility that islet cell progenitors do indeed reside within the pancreas and can be stimulated to differentiate into beta-like cells. Further studies aiming to isolate and characterize these progenitor cells hold great promise for the creation of a renewable source of new beta cells for beta cell replacement in diabetes.
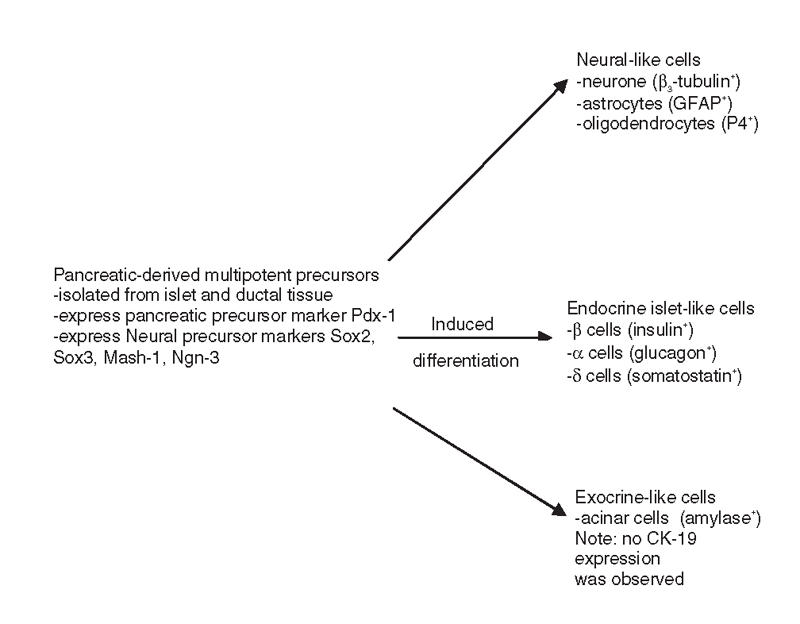
FIG. 2. Pancreatic-derived multipotent precursors (PMPs) can generate neural, pancreatic endocrine and pancreatic exocrine cells.
Discussion
Tremendous progress has been made in the field of islet regeneration in the past few years (Table 2). Nevertheless, the search for adult stem or progenitor cells in the pancreas is still in its early stages. Although a progenitor cell type in the adult mouse pancreas has been identified, there are still many unanswered questions. First, do similar pancreatic progenitors exist in the adult human pancreatic tissue? A clonal analysis is necessary to confirm this. If such a progenitor cell type does exist, then the next question is whether it is the only cell type responsible for pancreas recovery and islet neogenesis. Considering the small PMP population in the adult murine pancreas, as well as its limited self-renewal ability, it is possible that there may be another cell type responsible for islet neogenesis, whether it is another undiscovered stem or progenitor cell or an already identified pancreatic cell type. The contribution of beta cell replication in maintaining beta cell mass in the human islet remains a critical question. Lineage tracing studies to address these questions will prove extremely difficult in human cells but may be achieved with lentiviral vectors to express markers in cultured human pancreatic tissue.
Since it is possible to generate islet-like clusters from ductal tissue, it may be informative to examine the possible connection between islet-like clusters obtained by Cornelius and others and Bonner-Weir and others as well as the PMPs discovered by Seaberg and others. Did PMPs in ductal tissue give rise to these islet-like clusters, or did the surrounding tissue provide some trophic support for the differentiation of PMPs? What role do ductal cells play in this? The potential of ductal cells to transdifferentiate into islet cells needs to be more carefully evaluated. All ductal to islet cell transdifferentiation studies to date have been performed with impure ductal cells that might have been contaminated by other cell types, including beta cells. To eliminate this variable, the ductal cell purification process should be improved. Gmyr and others recently developed a method of rapidly purifying human ductal cells.38 Using fluorescent-activated cell sorting (FACS) and an antibody against pan-ductal surface carbohydrate antigen 19-9 (CA19-9), they obtained a ductal cell population with 97% purity and 76.7% viability. This isolation method may be a useful tool to show the potential role of ductal cells in islet neogenesis. The study by Dor and colleagues illustrated the use of lineage tracing as a tool to follow the development of pancreatic cells in vivo. In the future, this tracing method might be used to monitor the development of ductal cells to confirm whether new beta cells can arise through ductal cell differentiation. Meanwhile, the possibility of expanding beta cells as a source for transplantable insulin-secreting cells must now receive serious consideration.
Assessing the possibility of using islet-like clusters generated from ductal tissue to reverse type 1 diabetes should be a focus of research. Although mouse islet-like clusters may ameliorate diabetes in nonobese mice,24,25 human islet-like clusters could not engraft successfully in nude mice.28 In addition, culture conditions for these clusters need to be refined, so that these clusters can mature into islets with sufficient insulin production and regulated secretion of insulin in response to glucose.39 If human islet-like clusters are shown to have the ability to reverse diabetes and to secrete a sufficient amount of insulin, then they might be used as an alternative source of islet tissue, assuming that a sufficient number of cells can be generated in vitro.
Conclusion
Beta cell replacement by islet transplantation has provided new hope for a cure of type 1 diabetes. Several significant obstacles remain before islet transplantation as an accessible and permanent cure for all patients with type 1 diabetes can be estabilshed. Identifying, purifying and characterizing adult stem or progenitor cells that can differentiate into islet tissue is a critical step. Although available evidence indicates that pancreatic stem or progenitor cells are significant contributors to islet regeneration, the role of beta cell replication cannot be overlooked. New approaches are needed in the purification, differentiation and expansion of ductal tissue into insulin-secreting tissue to move this approach toward clinical reality. Recent advancements point to the great promise of research in this area and hopefully, with further analysis and research, an unlimited source of beta cells for islet transplantation will be found, moving a step closer to a cure for type 1 diabetes.
Accepted for publication Sept. 22, 2006.
Competing interests: None declared.
Correspondence to: Yu Huan Liao, Ike Barber Human Islet Transplant Laboratory, Department of Surgery, Faculty of Medicine, University of British Columbia, Suite 460 - 828 West 10th Ave, Vancouver BC V5Z 1L8; liaoamk@interchange.ubc.ca
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977-86. [DOI] [PubMed]
- 2.Fanelli CG, Porcellati F, Pampanelli S, et al. Insulin therapy and hypoglycaemia: the size of the problem. Diabetes Metab Res Rev 2004;20(Suppl 2):S32-S42. [DOI] [PubMed]
- 3.Helling TS. Surgical management of chronic pancreatitis and the role of islet cell autotransplantation. Curr Surg 2003;60:463-9. [DOI] [PubMed]
- 4.Oberholzer J, Mathe Z, Bucher P, et al. Islet autotransplantation after left pancreatectomy for non-enucleable insulinoma. Am J Transplant 2003;3:1302-7. [DOI] [PubMed]
- 5.Forster S, Liu X, Adam U, et al. Islet autotransplantation combined with pancreatectomy for treatment of pancreatic adenocarcinoma: a case report. Transplant Proc 2004;36:1125-6. [DOI] [PubMed]
- 6.Lacy PE. Pancreatic transplantation as a means of insulin delivery. Diabetes Care 1982;5(Suppl 1):93-7. [PubMed]
- 7.Trucco M. Regeneration of the pancreatic beta cell. J Clin Invest 2005;115:5-12. [DOI] [PMC free article] [PubMed]
- 8.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230-8. [DOI] [PubMed]
- 9.Lechner A, Habener JF. Stem/progenitor cells derived from adult tissues: potential for the treatment of diabetes mellitus. Am J Physiol Endocrinol Metab 2003;284:E259-66. [DOI] [PubMed]
- 10.Clarke D, Frisen J. Differentiation potential of adult stem cells. Curr Opin Genet Dev 2001;11:575-80. [DOI] [PubMed]
- 11.Poulsom R, Alison MR, Forbes SJ, et al. Adult stem cell plasticity. J Pathol 2002;197:441-56. [DOI] [PubMed]
- 12.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat Med 2004;10(Suppl):S42-S50. [DOI] [PubMed]
- 13.Fraser JK, Schreiber RE, Zuk PA, et al. Adult stem cell therapy for the heart. Int J Biochem Cell Biol 2004;36:658-66. [DOI] [PubMed]
- 14.Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci 2003;26:125-31. [DOI] [PubMed]
- 15.Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet 2004;364:203-5. [DOI] [PubMed]
- 16.Yang L, Li S, Hatch H, et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A 2002;11;99(12):8078-83. Epub. [DOI] [PMC free article] [PubMed]
- 17.Holland AM, Gonez LJ, Harrison LC. Progenitor cells in the adult pancreas. Diabetes Metab Res Rev 2004;20:13-27. [DOI] [PubMed]
- 18.Bouwens L, Lu WG, De Krijger R. Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia 1997;40:398-404. [DOI] [PubMed]
- 19.Leeson TS, Leeson R. Close association of centroacinar/ductular and insular cells in the rat pancreas. Histol Histopathol 1986;1:33-42. [PubMed]
- 20.Bertelli E, Regoli M, Orazioli D, et al. Association between islets of Langerhans and pancreatic ductal system in adult rat. Where endocrine and exocrine meet together? Diabetologia 2001;44:575-84. [DOI] [PubMed]
- 21.Bouwens L, Pipeleers DG. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia 1998;41:629-33. [DOI] [PubMed]
- 22.Jonsson J, Carlsson L, Edlund T, et al. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606-9. [DOI] [PubMed]
- 23.Heimberg H, Bouwens L, Heremans Y, et al. Adult human pancreatic duct and islet cells exhibit similarities in expression and differences in phosphorylation and complex formation of the homeodomain protein Ipf-1. Diabetes 2000;49:571-9. [DOI] [PubMed]
- 24.Cornelius JG, Tchernev V, Kao KJ, et al. In vitro-generation of islets in long-term cultures of pluripotent stem cells from adult mouse pancreas. Horm Metab Res 1997;29:271-7. [DOI] [PubMed]
- 25.Ramiya VK, Maraist M, Arfors KE, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 2000;6:278-82. [DOI] [PubMed]
- 26.Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 2000;97:7999-8004. [DOI] [PMC free article] [PubMed]
- 27.Katdare MR, Bhonde RR, Parab PB. Analysis of morphological and functional maturation of neoislets generated in vitro from pancreatic ductal cells and their suitability for islet banking and transplantation. J Endocrinol 2004;182:105-12. [DOI] [PubMed]
- 28.Gao R, Ustinov J, Pulkkinen M, et al. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 2003;52:2007-15. [DOI] [PubMed]
- 29.Bonner-Weir S, Toschi E, Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes 2004;5(Suppl 2):16-22. [DOI] [PubMed]
- 30.Dor Y, Brown J, Martinez OI, et al. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41-6. [DOI] [PubMed]
- 31.Beattie GM, Itkin-Ansari P, Cirulli V, et al. Sustained proliferation of PDX-1+ cells derived from human islets. Diabetes 1999;48:1013-9. [DOI] [PubMed]
- 32.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 2004;114:963-8. [DOI] [PMC free article] [PubMed]
- 33.Zaret K. Regenerative medicine: self-help for insulin cells. Nature 2004;429:30-1. [DOI] [PubMed]
- 34.Meier JJ, Bhushan A, Butler AE, et al. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005;48:2221-8. [DOI] [PubMed]
- 35.Petropavlovskaia M, Rosenberg L. Identification and characterization of small cells in the adult pancreas: potential progenitor cells? Cell Tissue Res 2002;310:51-8. [DOI] [PubMed]
- 36.Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes 2004;53:2143-52. [DOI] [PubMed]
- 37.Seaberg RM, Smukler SR, Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 2004;22:1115-24. [DOI] [PubMed]
- 38.Gmyr V, Belaich S, Muharram G, et al. Rapid purification of human ductal cells from human pancreatic fractions with surface antibody CA19-9. Biochem Biophys Res Commun 2004;320:27-33. [DOI] [PubMed]
- 39.Halban PA, Kahn SE, Lernmark A, et al. Gene and cell-replacement therapy in the treatment of type 1 diabetes: how high must the standards be set? Diabetes 2001;50:2181-91. [DOI] [PubMed]


