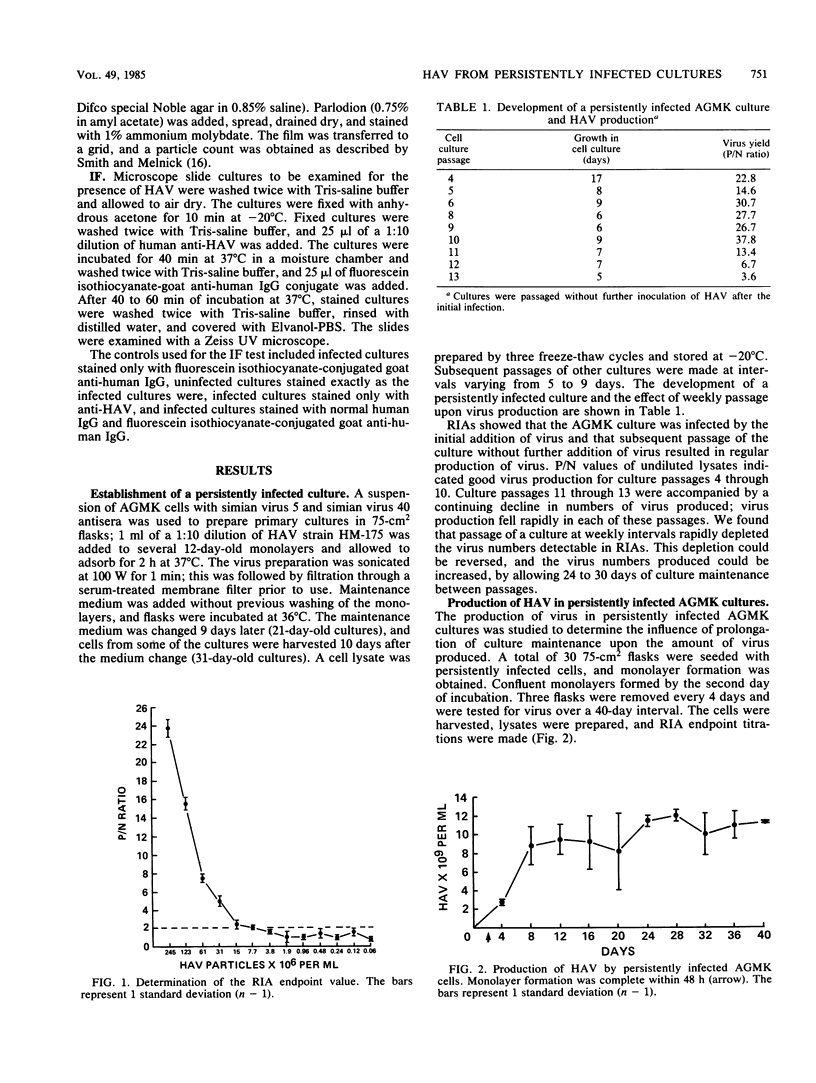
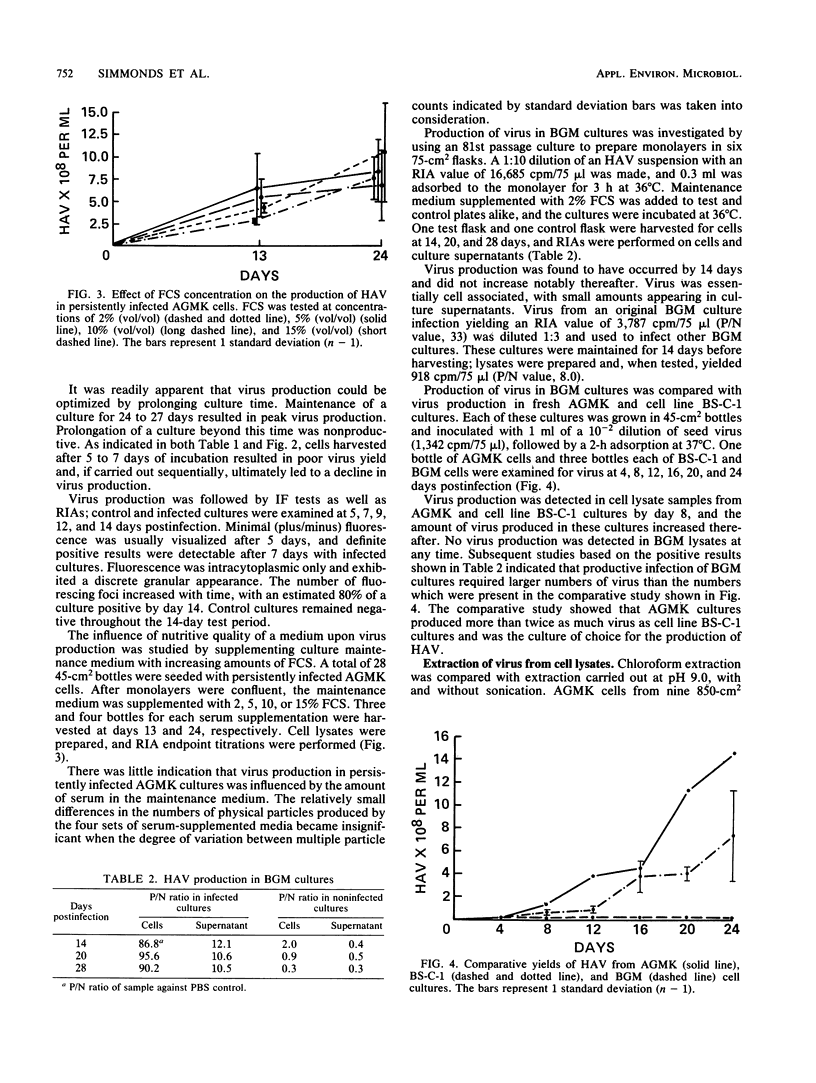
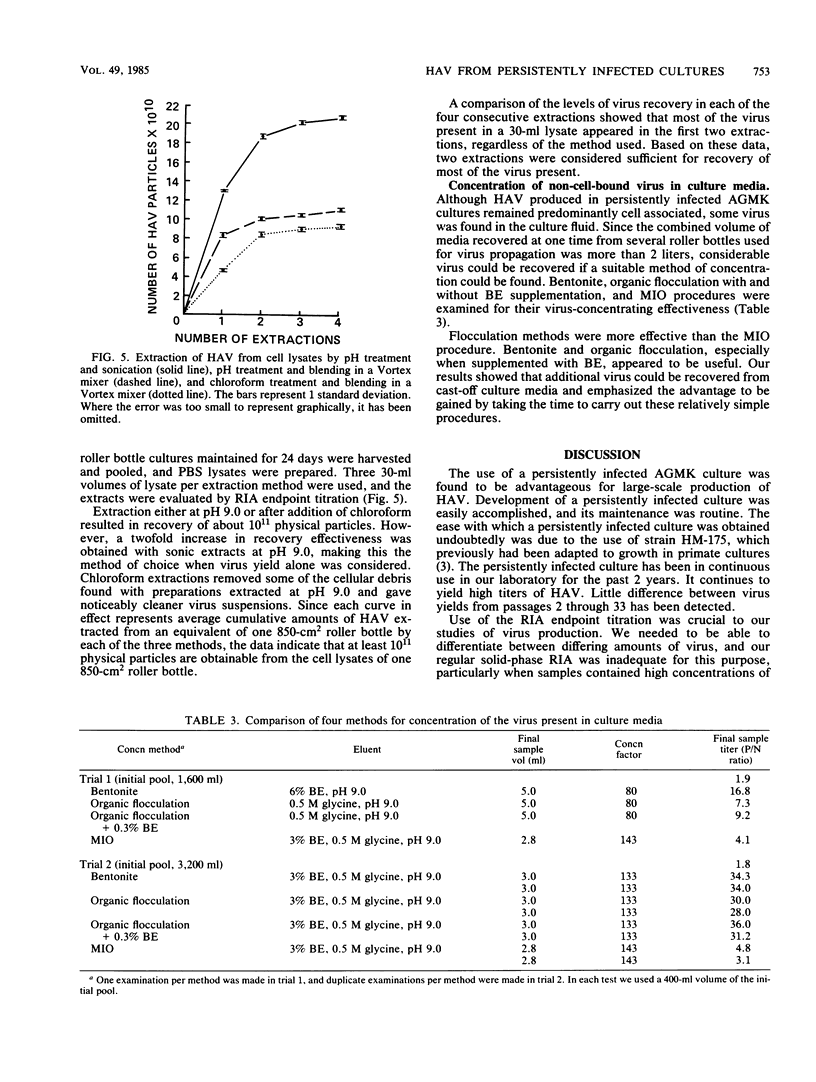
Abstract
Primary African green monkey kidney, continuous African green monkey kidney cell line BS-C-1, and buffalo green monkey kidney cultures were infected with a uniform inoculum of hepatitis A virus (HAV). Although both the cell line BS-C-1 and primary African green monkey kidney cultures produced useful amounts of virus, HAV was detected earlier and in greater quantities in primary African green monkey kidney cultures. A persistently infected primary African green monkey kidney culture was developed. The influence of incubation time (4 to 40 days) and concentration (2 to 15%) of fetal calf serum in the maintenance medium on production of HAV by this culture was examined. An incubation period of 24 to 28 days was found to be optimal; reducing this period led to decreased yields of HAV. No significant difference in the amount of HAV produced was observed with differing concentrations of fetal calf serum. Three different methods of extraction and the effect of multiple extractions on the recovery of HAV from cell lysates were examined. Sonication was a critical factor. Two extractions yielded more than 90% recoverable virus. Yields in excess of 10(11) physical particles of HAV per 850-cm2 roller bottle were routine. The total yield could be increased by concentrating the HAV present in spent maintenance medium by using bentonite or organic flocculation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binn L. N., Lemon S. M., Marchwicki R. H., Redfield R. R., Gates N. L., Bancroft W. H. Primary isolation and serial passage of hepatitis A virus strains in primate cell cultures. J Clin Microbiol. 1984 Jul;20(1):28–33. doi: 10.1128/jcm.20.1.28-33.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemer R. J., Feinstone S. M., Gust I. D., Purcell R. H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981 Apr;32(1):388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flehmig B. Hepatitis A virus in cell culture. II. Growth characteristics of hepatitis A virus in Frhk-4/R cells. Med Microbiol Immunol. 1981;170(2):73–81. doi: 10.1007/BF02122671. [DOI] [PubMed] [Google Scholar]
- Flehmig B. Hepatitis A-virus in cell culture: I. propagation of different hepatitis A-virus isolates in a fetal rhesus monkey kidney cell line (Frhk-4). Med Microbiol Immunol. 1980;168(4):239–248. doi: 10.1007/BF02121807. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Vallbracht A., Wurster G. Hepatitis A virus in cell culture. III. Propagation of hepatitis A virus in human embryo kidney cells and human embryo fibroblast strains. Med Microbiol Immunol. 1981;170(2):83–89. doi: 10.1007/BF02122672. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss-Müller V., Deinhardt F. Effect of hepatitis A virus infection on cell metabolism in vitro. Proc Soc Exp Biol Med. 1984 Jan;175(1):10–15. doi: 10.3181/00379727-175-41757. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Frösner G. G., Deinhardt F. Propagation of hepatitis A virus in human embryo fibroblasts. J Med Virol. 1981;7(3):233–239. doi: 10.1002/jmv.1890070308. [DOI] [PubMed] [Google Scholar]
- Hollinger F. B., Bradley D. W., Maynard J. E., Dreesman G. R., Melnick J. L. Detection of hepatitis A viral antigen by radioimmunoassay. J Immunol. 1975 Nov;115(5):1464–1466. [PubMed] [Google Scholar]
- Kojima H., Shibayama T., Sato A., Suzuki S., Ichida F., Hamada C. Propagation of human hepatitis A virus in conventional cell lines. J Med Virol. 1981;7(4):273–286. doi: 10.1002/jmv.1890070404. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- Rao V. C., Waghmare S. V., Lakhe S. B. Detection of viruses in drinking water by concentration on magnetic iron oxide. Appl Environ Microbiol. 1981 Sep;42(3):421–426. doi: 10.1128/aem.42.3.421-426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. Electron microscopic counting of virus particles by sedimentation on aluminized grids. J Immunol. 1962 Aug;89:279–284. [PubMed] [Google Scholar]
- Widell A., Hansson B. G., Nordenfelt E. A microcarrier cell culture system for large scale production of hepatitis A virus. J Virol Methods. 1984 Feb;8(1-2):63–71. doi: 10.1016/0166-0934(84)90041-7. [DOI] [PubMed] [Google Scholar]


