Abstract
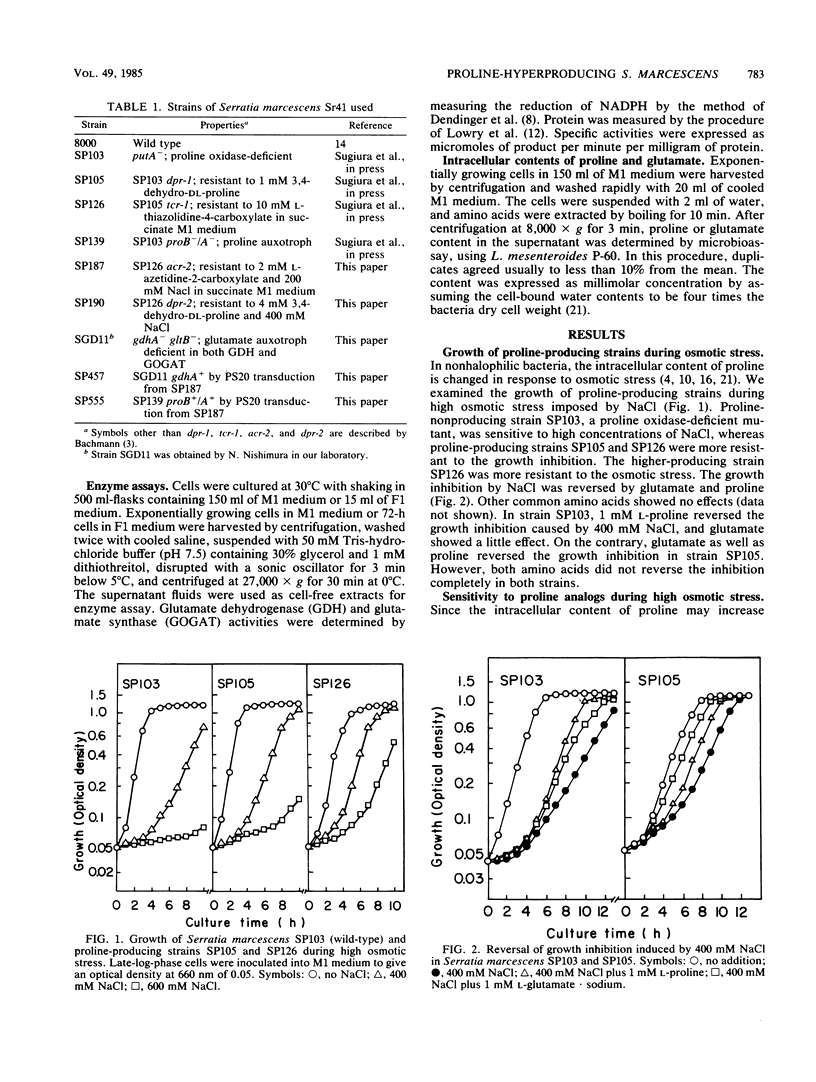
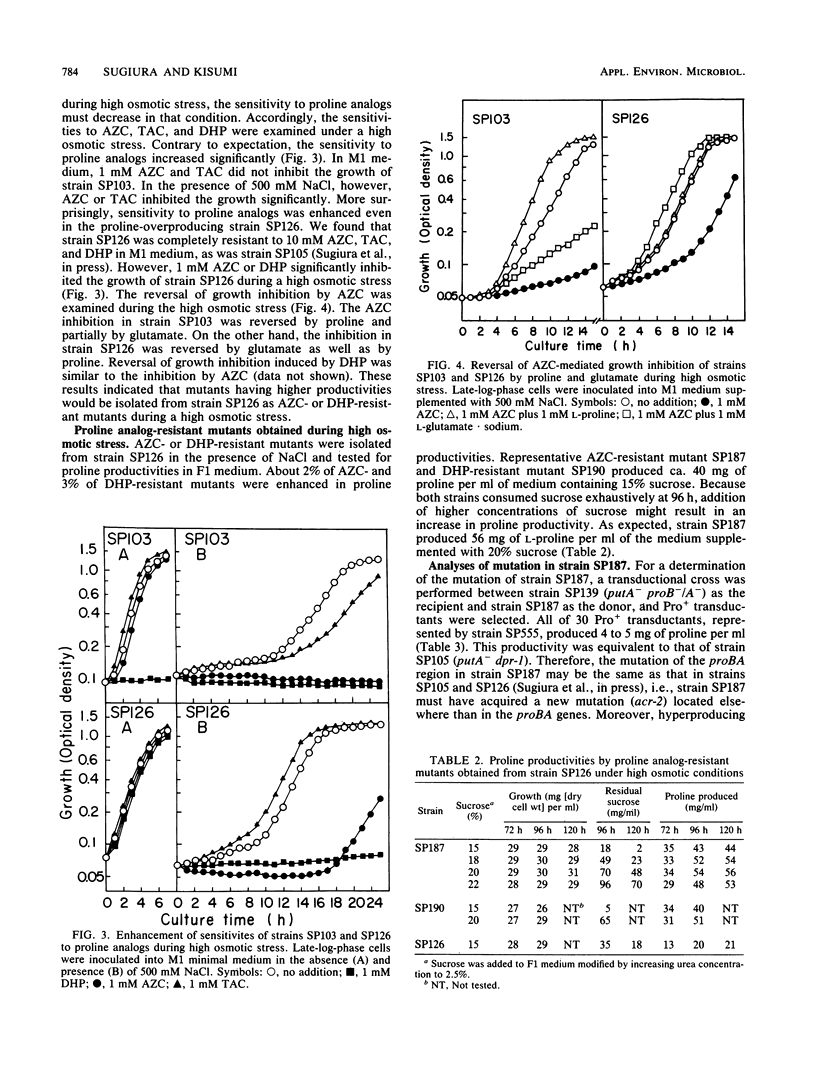
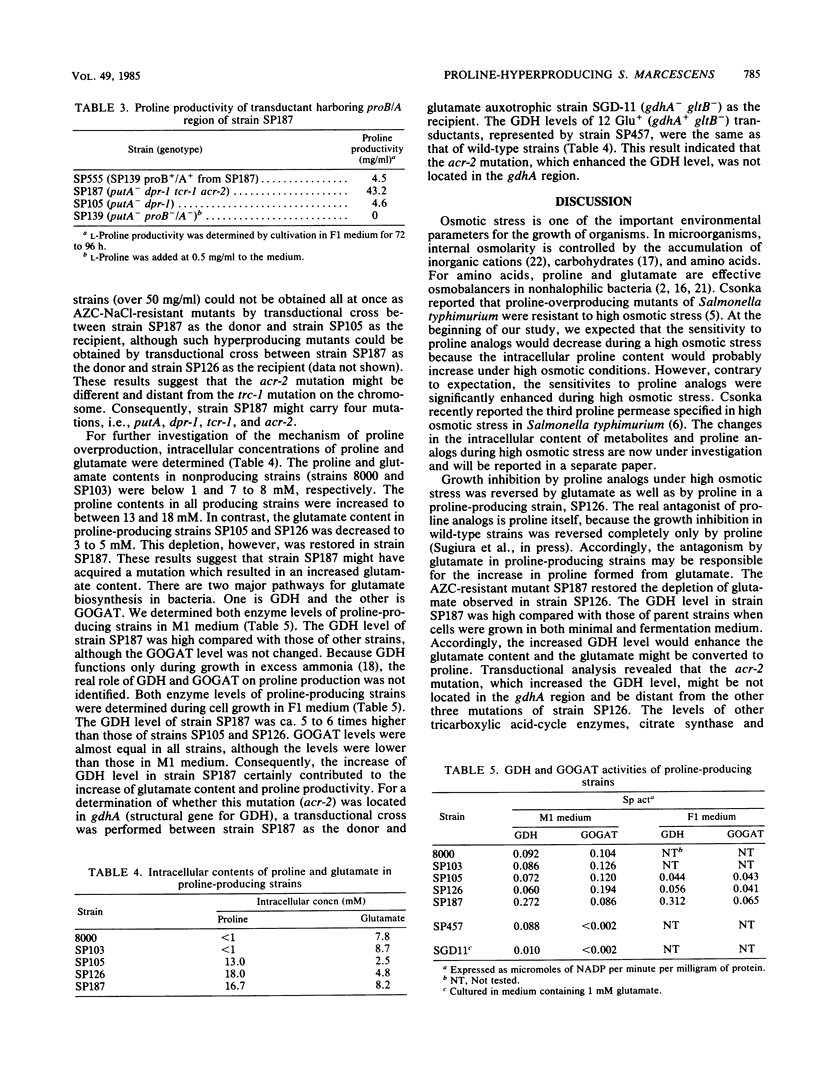
Proline-producing strains of Serratia marcescens were more osmotolerant than wild-type strains. Growth inhibition by proline analogs was significantly enhanced by increasing the osmotic stress of the medium. Mutants resistant to azetidine-2-carboxylate were derived from a proline-producing strain, SP126, under a high osmotic condition. One of the mutants, strain SP187, produced 56 mg of L-proline per ml of medium containing sucrose and urea. This amount was ca. 3 times larger than that produced by strain SP126. The intracellular glutamate content which decreased in strain SP126 was restored in strain SP187. The glutamate dehydrogenase level of strain SP187 was 5 times higher than that of strain SP126.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E., Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- Anderson C. B., Witter L. D. Glutamine and proline accumulation by Staphylococcus aureus with reduction in water activity. Appl Environ Microbiol. 1982 Jun;43(6):1501–1503. doi: 10.1128/aem.43.6.1501-1503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. A third L-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol. 1982 Sep;151(3):1433–1443. doi: 10.1128/jb.151.3.1433-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182(1):82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendinger S. M., Patil L. G., Brenchley J. E. Salmonella typhimurium mutants with altered glutamate dehydrogenase and glutamate synthase activities. J Bacteriol. 1980 Jan;141(1):190–198. doi: 10.1128/jb.141.1.190-198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayzer D. J. Sub-cloning of the wild-type proAB region of the Escherichia coli genome. J Gen Microbiol. 1983 Oct;129(10):3215–3225. doi: 10.1099/00221287-129-10-3215. [DOI] [PubMed] [Google Scholar]
- Koujima I., Hayashi H., Tomochika K., Okabe A., Kanemasa Y. Adaptational change in proline and water content of Staphylococcus aureus after alteration of environmental salt concentration. Appl Environ Microbiol. 1978 Mar;35(3):467–470. doi: 10.1128/aem.35.3.467-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahan M. J., Csonka L. N. Genetic analysis of the proBA genes of Salmonella typhimurium: physical and genetic analyses of the cloned proB+ A+ genes of Escherichia coli and of a mutant allele that confers proline overproduction and enhanced osmotolerance. J Bacteriol. 1983 Dec;156(3):1249–1262. doi: 10.1128/jb.156.3.1249-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Hosogaya S., Suzuki K., Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975 Feb;19(1):35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T., Hosogaya S. A generalized transducing phage of Serratia marcescens. Jpn J Microbiol. 1973 Nov;17(6):473–479. doi: 10.1111/j.1348-0421.1973.tb00933.x. [DOI] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Roller S. D., Anagnostopoulos G. D. Accumulation of carbohydrate by Escherichia coli B/r/1 during growth at low water activity. J Appl Bacteriol. 1982 Jun;52(3):425–434. doi: 10.1111/j.1365-2672.1982.tb05073.x. [DOI] [PubMed] [Google Scholar]
- Rossi J. J., Vender J., Berg C. M., Coleman W. H. Partial purification and some properties of delta1-pyrroline-5-carboxylate reductase from Escherichia coli. J Bacteriol. 1977 Jan;129(1):108–114. doi: 10.1128/jb.129.1.108-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Kisumi M. Stabilization of a histidine-producing strain of Serratia marcescens. Appl Environ Microbiol. 1984 Jul;48(1):43–47. doi: 10.1128/aem.48.1.43-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Potassium transport and the relationship between intracellular potassium concentration and amino acid uptake by cells of a marine pseudomonad. J Bacteriol. 1974 Nov;120(2):598–603. doi: 10.1128/jb.120.2.598-603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]


