Abstract
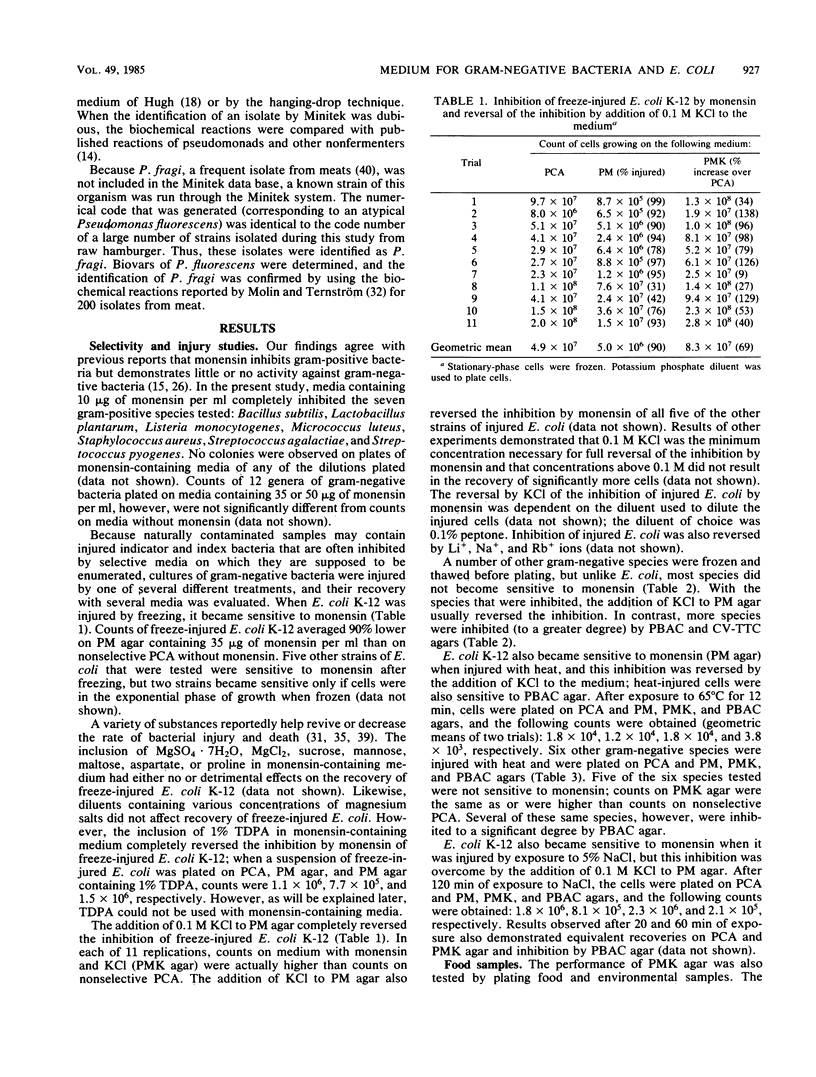
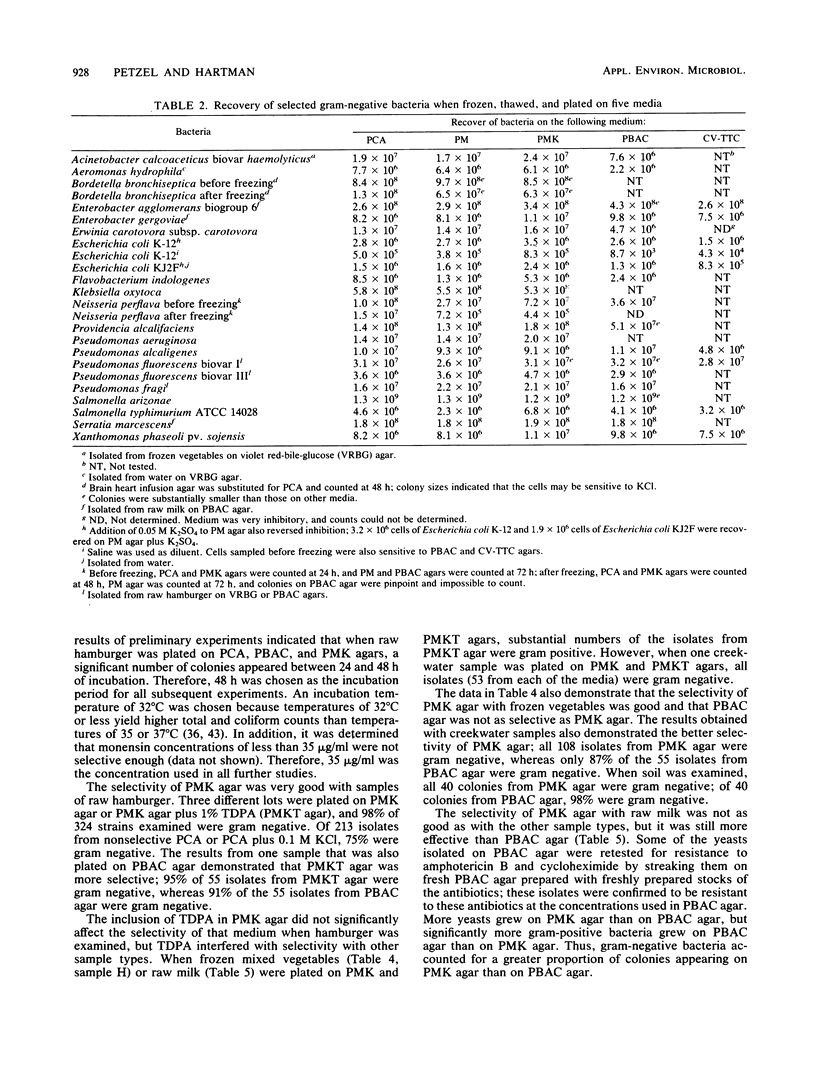
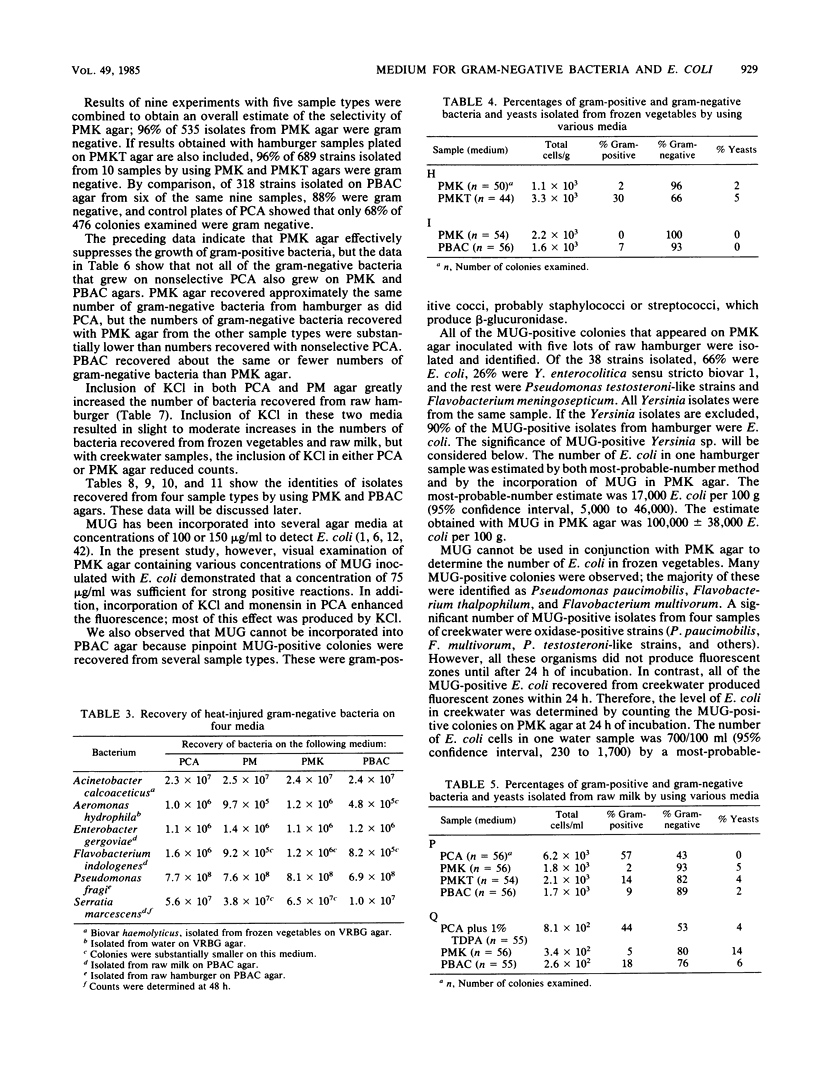
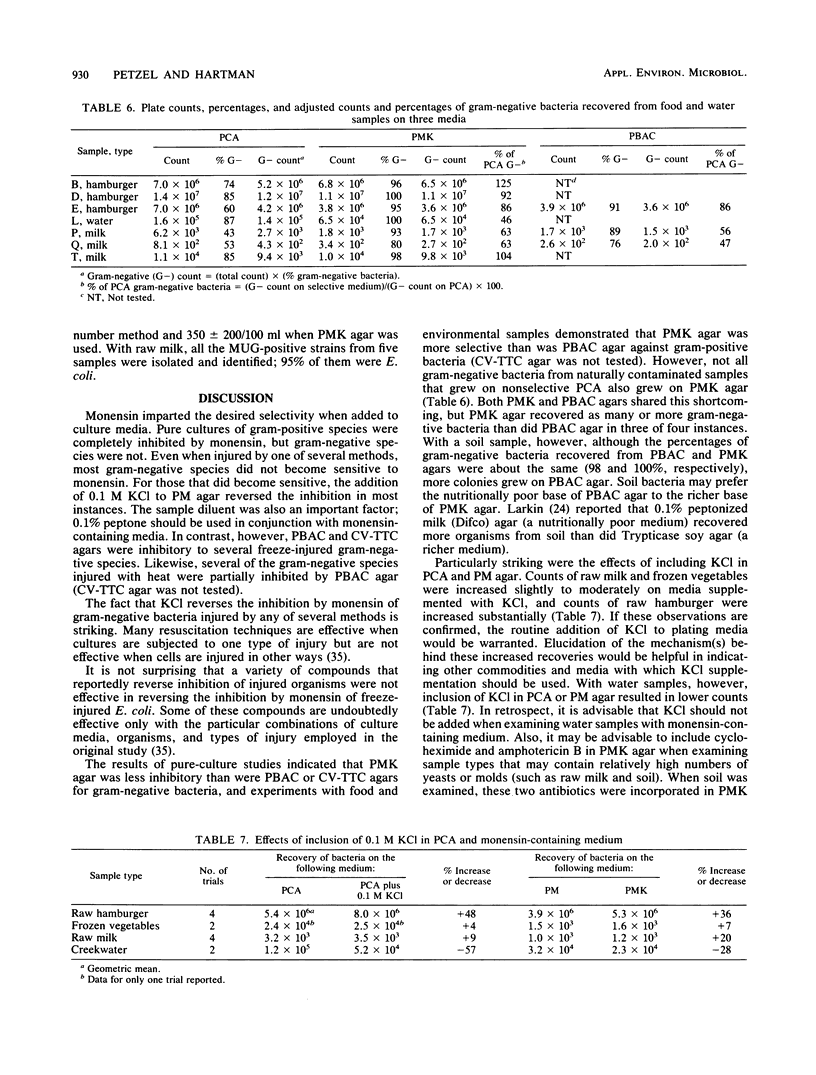
Plate count-monensin-KCl (PMK) agar, for enumeration of both gram-negative bacteria and Escherichia coli, is composed of (per liter) 23.5 g of plate count agar, 35 mg of monensin, 7.5 g of KCl, and 75 mg of 4-methylumbelliferyl-beta-D-glucuronide (MUG). Monensin was added after the medium was sterilized. The diluent of choice for use with PMK agar was 0.1% peptone (pH 6.8); other diluents were unsatisfactory. Gram-negative bacteria (selected for by the ionophore monensin) can be used to judge the general quality or sanitary history of a commodity. E. coli (differentiated by its ability to hydrolyze the fluorogenic compound MUG) can be used to assess the safety of a commodity in regard to the possible presence of enteric pathogens. Pure-culture studies demonstrated that monensin completely inhibited gram-positive bacteria and had little or no effect on gram-negative bacteria. When gram-negative bacteria were injured by one of several methods, a few species (including E. coli) became sensitive to monensin; this sensitivity was completely reversed in most instances by the inclusion of KCl in the medium. When PMK agar was tested with food and environmental samples, 96% of 535 isolates were gram negative; approximately 68% of colonies from nonselective medium were gram negative. PMK agar was more selective than two other media against gram-positive bacteria and was less inhibitory for gram-negative bacteria. However, with water samples, KCl had an inhibitory effect on gram-negative bacteria, and it should therefore be deleted from monensin-containing medium for water analysis.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cyzeska F. J., Seiter J. A., Marks S. N., Jay J. M. Culture medium for selective isolation and enumeration of Gram-negative bacteria from ground meats. Appl Environ Microbiol. 1981 Aug;42(2):303–307. doi: 10.1128/aem.42.2.303-307.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén G., Linde A. Screening plate method for detection of bacterial beta-glucuronidase. Appl Microbiol. 1973 Dec;26(6):863–866. doi: 10.1128/am.26.6.863-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty R. H., Shaw B. G., Roberts T. A. Microbial and chemical changes in chill-stored red meats. Soc Appl Bacteriol Symp Ser. 1983;11:151–178. [PubMed] [Google Scholar]
- Donnelly L. S., Hartman P. A. Gentamicin-based medium for the isolation of group D streptococci and application of the medium to water analysis. Appl Environ Microbiol. 1978 Mar;35(3):576–581. doi: 10.1128/aem.35.3.576-581.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutkaand B. J., Tobin S. E. Study on the efficiency of four procedures for enumerating coliforms in water. Can J Microbiol. 1976 May;22(5):630–635. doi: 10.1139/m76-093. [DOI] [PubMed] [Google Scholar]
- Evans T. M., Seidler R. J., LeChevallier M. W. Impact of verification media and resuscitation on accuracy of the membrane filter total coliform enumeration technique. Appl Environ Microbiol. 1981 May;41(5):1144–1151. doi: 10.1128/aem.41.5.1144-1151.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. M., Waarvick C. E., Seidler R. J., LeChevallier M. W. Failure of the most-probable-number technique to detect coliforms in drinking water and raw water supplies. Appl Environ Microbiol. 1981 Jan;41(1):130–138. doi: 10.1128/aem.41.1.130-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P. C., Hartman P. A. Fluorogenic assays for immediate confirmation of Escherichia coli. Appl Environ Microbiol. 1982 Jun;43(6):1320–1329. doi: 10.1128/aem.43.6.1320-1329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Bülow P. Rapid diagnosis of Enterobacteriaceae. I. Detection of bacterial glycosidases. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):245–251. doi: 10.1111/j.1699-0463.1976.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Kodaka H., Armfield A. Y., Lombard G. L., Dowell V. R., Jr Practical procedure for demonstrating bacterial flagella. J Clin Microbiol. 1982 Nov;16(5):948–952. doi: 10.1128/jcm.16.5.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J. M. Peptonized milk as an enumeration medium for soil bacteria. Appl Microbiol. 1972 May;23(5):1031–1032. doi: 10.1128/am.23.5.1031-1032.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minor L., Buissière J., Novel G., Novel M. Relation entre le sérotype et l'activité beta-glucuronidasique chez les Salmonella. Ann Microbiol (Paris) 1978 Aug-Sep;129B(2):155–165. [PubMed] [Google Scholar]
- Massenti M. F., Scarlata G., Nastasi A. Beta-glucuronidase activity in Enterobacteriaceae. Boll Ist Sieroter Milan. 1981;60(1):26–30. [PubMed] [Google Scholar]
- McDonald L. C., Hackney C. R., Ray B. Enhanced recovery of injured Escherichia coli by compounds that degrade hydrogen peroxide or block its formation. Appl Environ Microbiol. 1983 Feb;45(2):360–365. doi: 10.1128/aem.45.2.360-365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin G., Ternström A. Numerical taxonomy of psychrotrophic pseudomonads. J Gen Microbiol. 1982 Jun;128(6):1249–1264. doi: 10.1099/00221287-128-6-1249. [DOI] [PubMed] [Google Scholar]
- Ray B., Janssen D. W., Busta F. F. Characterization of the repair of injury induced by freezing Salmonella anatum. Appl Microbiol. 1972 Apr;23(4):803–809. doi: 10.1128/am.23.4.803-809.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepeta R. W., Edberg S. C. Methylumbelliferyl-beta-D-glucuronide-based medium for rapid isolation and identification of Escherichia coli. J Clin Microbiol. 1984 Feb;19(2):172–174. doi: 10.1128/jcm.19.2.172-174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


