Abstract
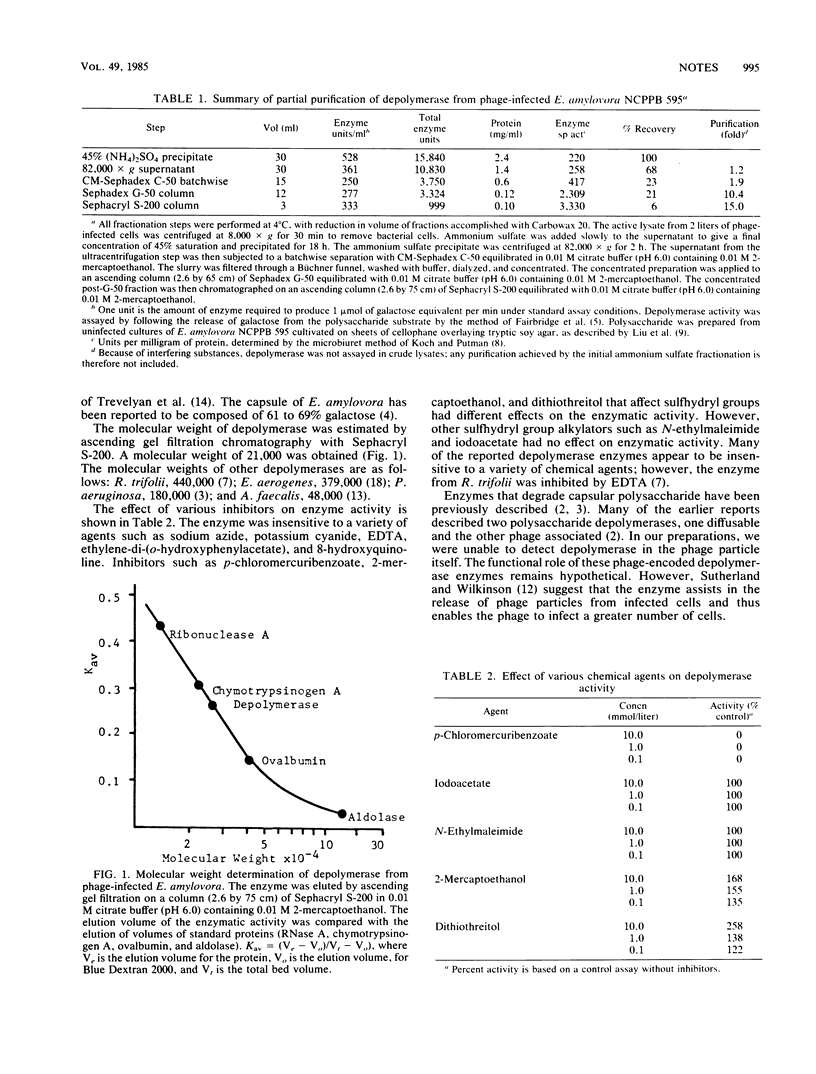
Erwinia amylovora infected with bacteriophage ERA103 produced an enzyme which degraded the extracellular polysaccharide of noninfected cells. The depolymerase enzyme was purified 15-fold by a procedure which included ammonium sulfate precipitation, ultracentrifugation, CM-Sephadex batchwise separation, Sephadex G-50 column chromatography, and Sephacryl S-200 column chromatography. The enzyme had a molecular weight of approximately 21,000 and a pH optimum of 6.0. Activity was enhanced by supplements of 2-mercaptoethanol or dithiothreitol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., PARK B. H. An enzyme produced by a phage-host cell system. II. The properties of the polysaccharide depolymerase. Virology. 1956 Dec;2(6):719–736. doi: 10.1016/0042-6822(56)90054-x. [DOI] [PubMed] [Google Scholar]
- Ackermann H. W., Audurier A., Berthiaume L., Jones L. A., Mayo J. A., Vidaver A. K. Guidelines for bacteriophage characterization. Adv Virus Res. 1978;23:1–24. doi: 10.1016/s0065-3527(08)60096-2. [DOI] [PubMed] [Google Scholar]
- Bartell P. F., Lam G. K., Orr T. E. Purification and properties of polysaccharide depolymerase associated with phage-infected Pseudomonas aeruginosa. J Biol Chem. 1968 May 10;243(9):2077–2080. [PubMed] [Google Scholar]
- FAIRBRIDGE R. A., WILLIS K. J., BOOTH R. G. The direct colorimetric estimation of reducing sugars and other reducing substances with tetrazolium salts. Biochem J. 1951 Sep;49(4):423–427. doi: 10.1042/bj0490423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Putnam S. L. Sensitive biuret method for determination of protein in an impure system such as whole bacteria. Anal Biochem. 1971 Nov;44(1):239–245. doi: 10.1016/0003-2697(71)90366-6. [DOI] [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Highly specific bacteriophage-associated polysaccharide hydrolases for Klebsiella aerogenes type 8. J Gen Microbiol. 1976 May;94(1):211–216. doi: 10.1099/00221287-94-1-211. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W., Wilkinson J. F. Depolymerases for bacterial exopolysaccharides obtained from phage-infected bacteria. J Gen Microbiol. 1965 Jun;39(3):373–383. doi: 10.1099/00221287-39-3-373. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tanio T., Fukui T., Shirakura Y., Saito T., Tomita K., Kaiho T., Masamune S. An extracellular poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. Eur J Biochem. 1982 May;124(1):71–77. doi: 10.1111/j.1432-1033.1982.tb05907.x. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Schuster M. L. Characterization of Xanthomonas phaseoli Bacteriophages. J Virol. 1969 Sep;4(3):300–308. doi: 10.1128/jvi.4.3.300-308.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967 Nov;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yurewicz E. C., Ghalambor M. A., Duckworth D. H., Heath E. C. Catalytic and molecular properties of a phage-induced capsular polysaccharide depolymerase. J Biol Chem. 1971 Sep 25;246(18):5607–5616. [PubMed] [Google Scholar]


