Abstract
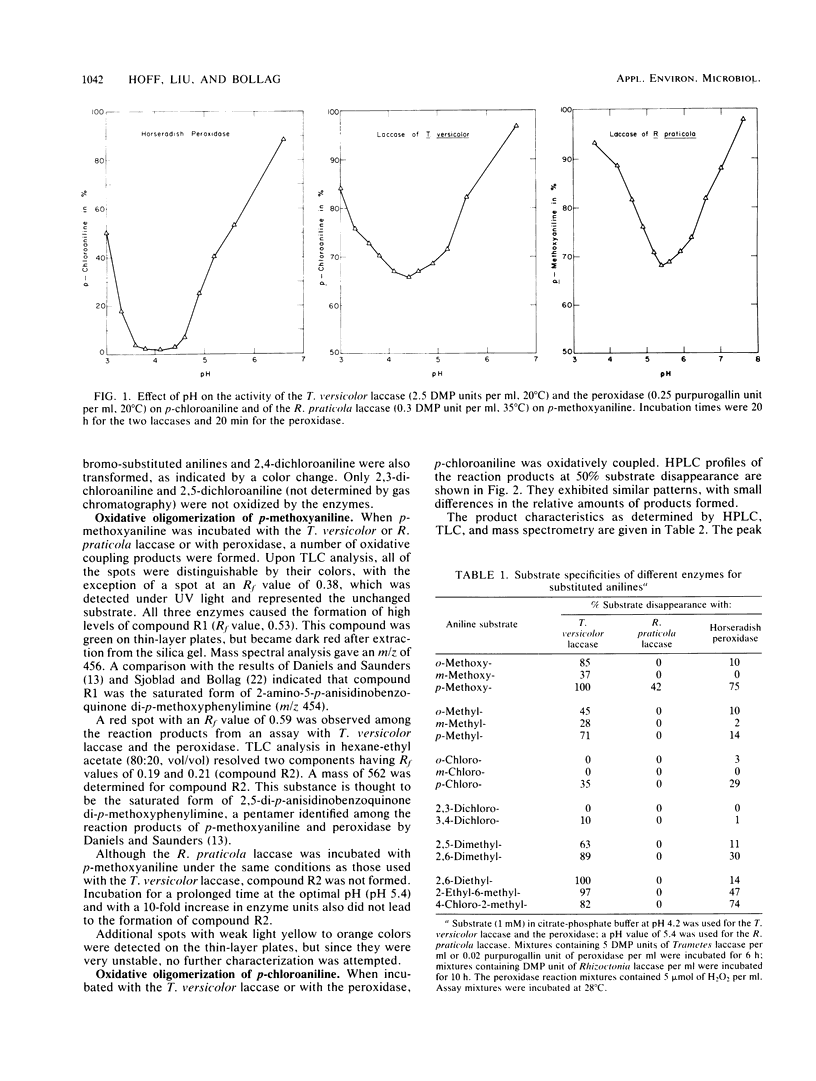
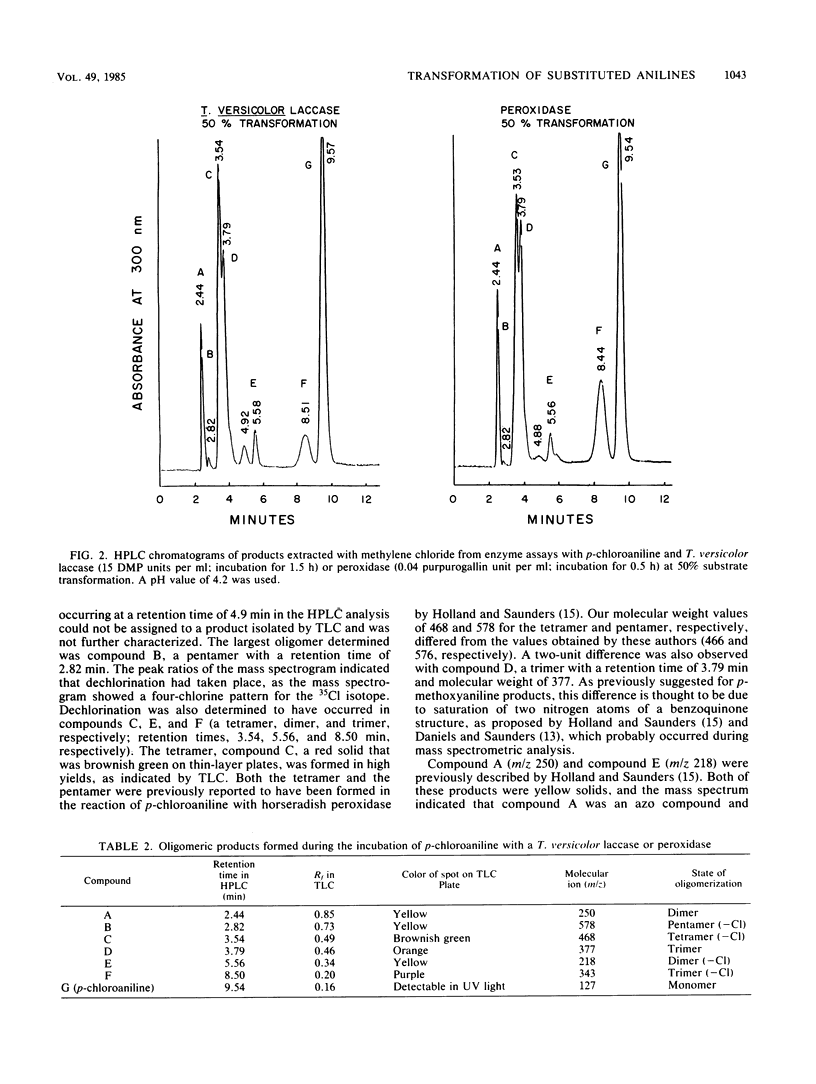
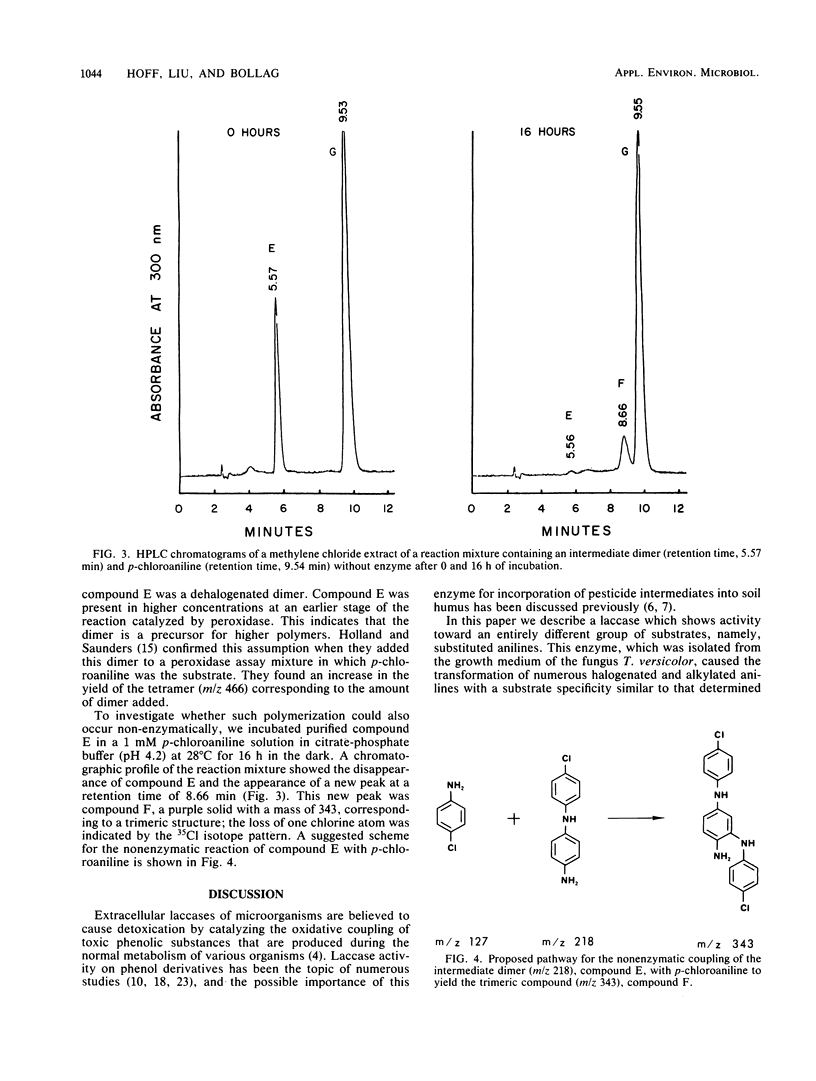
The laccase of the fungus Trametes versicolor was able to polymerize various halogen-, alkyl-, and alkoxy-substituted anilines, showing substrate specificity similar to that of horseradish peroxidase, whereas the laccase of Rhizoctonia praticola was active only with p-methoxyaniline. The substrate specificities of the enzymes were determined by using gas chromatography to measure the decrease in substrate concentration during incubation. With p-chloroaniline as the substrate, the peroxidase and the Trametes laccase showed maximum activity near pH 4.2. The transformation of this substrate gave rise to a number of oligomers, ranging from dimers to pentamers, as determined by mass spectrometry. The product profiles obtained by high-pressure liquid chromatography were similar for the two enzymes. A chemical reaction was observed between p-chloroaniline and an enzymatically formed dimer, resulting in the formation of a trimer. All three enzymes oxidized p-methoxyaniline to 2-amino-5-p-anisidinobenzoquinone di-p-methoxyphenylimine, but only the T. versicolor laccase and the peroxidase caused the formation of a pentamer (2,5-di-p-anisidinobenzoquinone di-p-methoxyphenylimine). Our results demonstrate that in addition to horseradish peroxidase, a T. versicolor laccase can also polymerize aniline derivatives.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartha R., Linke H. A., Pramer D. Pesticide transformations: production of chloroazobenzenes from chloroanilines. Science. 1968 Aug 9;161(3841):582–583. doi: 10.1126/science.161.3841.582. [DOI] [PubMed] [Google Scholar]
- Bollag J. M., Leonowicz A. Comparative studies of extracellular fungal laccases. Appl Environ Microbiol. 1984 Oct;48(4):849–854. doi: 10.1128/aem.48.4.849-854.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag J. M., Sjoblad R. D., Liu S. Y. Characterization of an enzyme from Rhizoctonia praticola which polymerizes phenolic compounds. Can J Microbiol. 1979 Feb;25(2):229–233. doi: 10.1139/m79-035. [DOI] [PubMed] [Google Scholar]
- Bordeleau L. M., Bartha R. Biochemical transformations of herbicide-derived anilines: requirements of molecular configuration. Can J Microbiol. 1972 Dec;18(12):1873–1882. doi: 10.1139/m72-292. [DOI] [PubMed] [Google Scholar]
- Chisaka H., Kearney P. C. Metabolism of propanil in soils. J Agric Food Chem. 1970 Sep-Oct;18(5):854–858. doi: 10.1021/jf60171a032. [DOI] [PubMed] [Google Scholar]
- Holland V. R., Saunders B. C. Studies in peroxidase action. 18. The oxidation of 4-chloraniline--further studies. Tetrahedron. 1968 Jan;24(2):585–588. doi: 10.1016/0040-4020(68)88008-1. [DOI] [PubMed] [Google Scholar]
- KAUFMAN D. D., KEARNEY P. C. MICROBIAL DEGRADATION OF ISOPROPYL-N-3 -CHLOROPHENYLCARBAMATE AND 2-CHLOROETHYL-N-3-CHLOROPHENYLCARBAMATE. Appl Microbiol. 1965 May;13:443–446. doi: 10.1128/am.13.3.443-446.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb H. B., Still C. C. Herbicide metabolism in plants: specificity of peroxidases for aniline substrates. Plant Physiol. 1969 Dec;44(12):1672–1673. doi: 10.1104/pp.44.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblad R. D., Bollag J. M. Oxidative coupling of aromatic pesticide intermediates by a fungal phenol oxidase. Appl Environ Microbiol. 1977 Apr;33(4):906–910. doi: 10.1128/aem.33.4.906-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alfen N. K., Kosuge T. Metabolism of the fungicide 2,6-dichloro-4-nitroaniline in soil. J Agric Food Chem. 1976 May-Jun;24(3):584–588. doi: 10.1021/jf60205a029. [DOI] [PubMed] [Google Scholar]


