Abstract
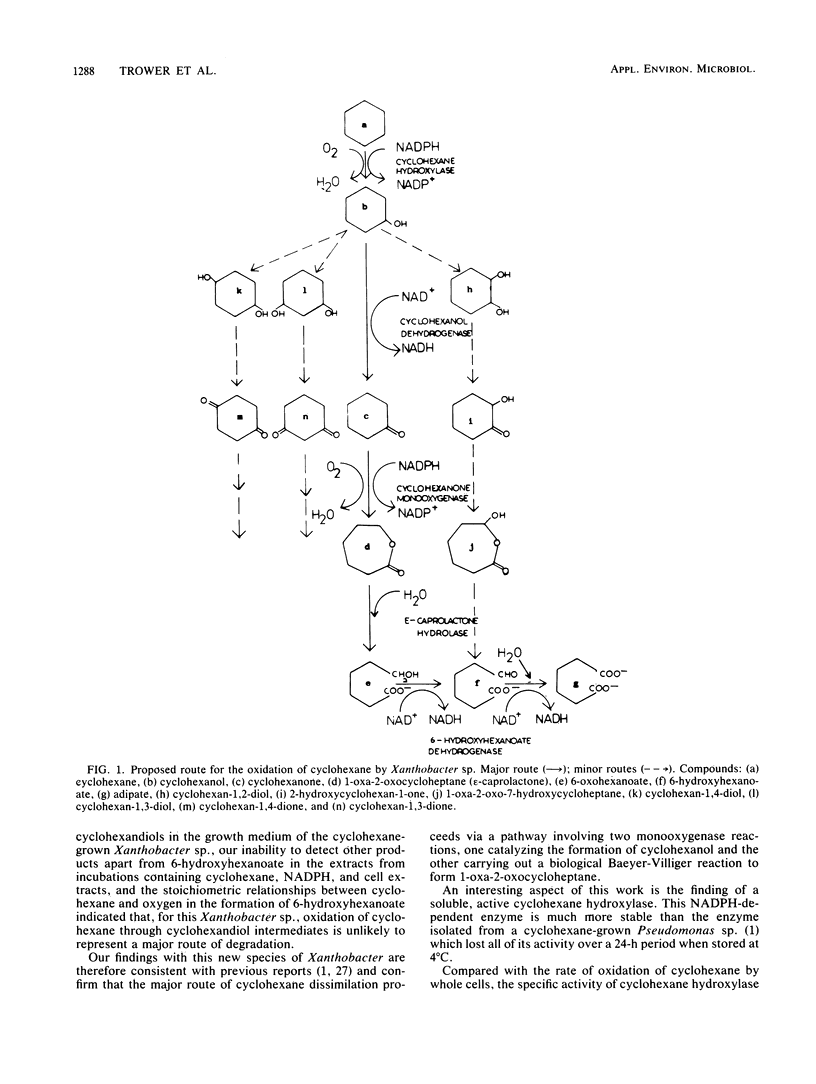
An unusual Xanthobacter sp., capable of independent growth on cyclohexane as the sole source of carbon and energy, has been isolated from soil by using classical enrichment techniques. The mean generation time for growth on cyclohexane was 6 h. The microorganism showed a limited ability to utilize hydrocarbons, with only alicyclic hydrocarbons closely related to cyclohexane supporting growth. Ultrastructural studies indicated the presence of electron-transparent vesicles in the cyclohexane-grown Xanthobacter sp., but the presence of complex intracytoplasmic membranes could not be identified. A soluble inducible enzyme capable of oxidizing cyclohexane was identified in cell extracts. This enzyme had a pH optimum of 6.5, an absolute specificity for NADPH, and a stoichiometric requirement for molecular O2 which was consistent with the formation of cyclohexanol. The enzyme showed no activity towards straight chain alkanes and only a limited activity towards unsaturated ring compounds. Enzymatic studies with cell extracts have indicated the main route of metabolism of cyclohexane by this Xanthobacter sp. to proceed via cyclohexane → cyclohexanol → cyclohexanone → 1-oxa-2-oxocycloheptane (ε-caprolactone) → 6-hydroxyhexanoate (6-hydroxycaproate) → → adipic acid. Alternative routes involving initial double hydroxylation of the cyclohexane ring may operate fortuituously but are unlikely to represent major pathways for the dissimilation of cyclohexane by this microorganism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUNDSTONE H. A. Paper chromatography of organic acids. Nature. 1963 Jan 26;197:377–377. doi: 10.1038/197377a0. [DOI] [PubMed] [Google Scholar]
- Beam H. W., Perry J. J. Co-metabolism as a factor in microbial degradation of cycloparaffinic hydrocarbons. Arch Mikrobiol. 1973 Apr 8;91(1):87–90. doi: 10.1007/BF00409542. [DOI] [PubMed] [Google Scholar]
- CAIN R. B. The metabolism of protocatechuic acid by a vibrio. Biochem J. 1961 May;79:298–312. doi: 10.1042/bj0790298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Trudgill P. W. The metabolism of trans-cyclohexan-1,2-diol by an Acinetobacter species. Eur J Biochem. 1977 Mar 15;74(1):115–127. doi: 10.1111/j.1432-1033.1977.tb11373.x. [DOI] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- Kennedy R. S., Finnerty W. R. Microbial assimilation of hydrocarbons. II. Intracytoplasmic membrane induction in Acinetobacter sp. Arch Microbiol. 1975;102(2):85–90. doi: 10.1007/BF00428350. [DOI] [PubMed] [Google Scholar]
- Murray J. R., Scheikowski T. A., MacRae I. C. Utilization of cyclohexanone and related substances by a Nocardia sp. Antonie Van Leeuwenhoek. 1974;40(1):17–24. doi: 10.1007/BF00394549. [DOI] [PubMed] [Google Scholar]
- Norris D. B., Trudgill P. W. The metabolism of cyclohexanol by Nocardia globerula CL1. Biochem J. 1971 Feb;121(3):363–370. doi: 10.1042/bj1210363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OOYAMA J., FOSTER J. W. BACTERIAL OXIDATION OF CYCLOPARAFFINIC HYDROCARBONS. Antonie Van Leeuwenhoek. 1965;31:45–65. doi: 10.1007/BF02045875. [DOI] [PubMed] [Google Scholar]
- Scott C. C., Finnerty W. R. A comparative analysis of the ultrastructure of hydrocarbon-oxidizing micro-organisms. J Gen Microbiol. 1976 Jun;94(2):342–350. doi: 10.1099/00221287-94-2-342. [DOI] [PubMed] [Google Scholar]
- YUGARI Y. Metabolism of cyclohexane-diol-(1,2)-trans by a soil bacterium. Biken J. 1961 Sep;4:197–207. [PubMed] [Google Scholar]
- de Klerk H., van der Linden A. C. Bacterial degradation of cyclohexane. Participation of a co-oxidation reaction. Antonie Van Leeuwenhoek. 1974;40(1):7–15. doi: 10.1007/BF00394548. [DOI] [PubMed] [Google Scholar]



