Abstract
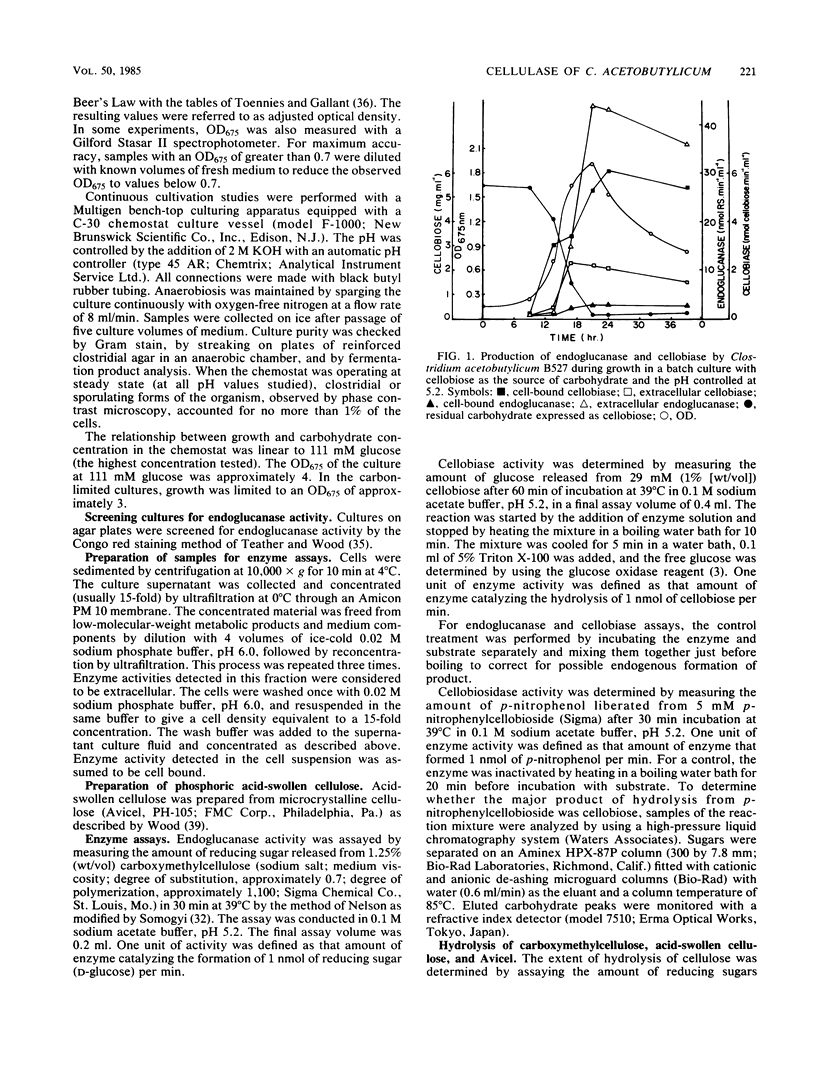
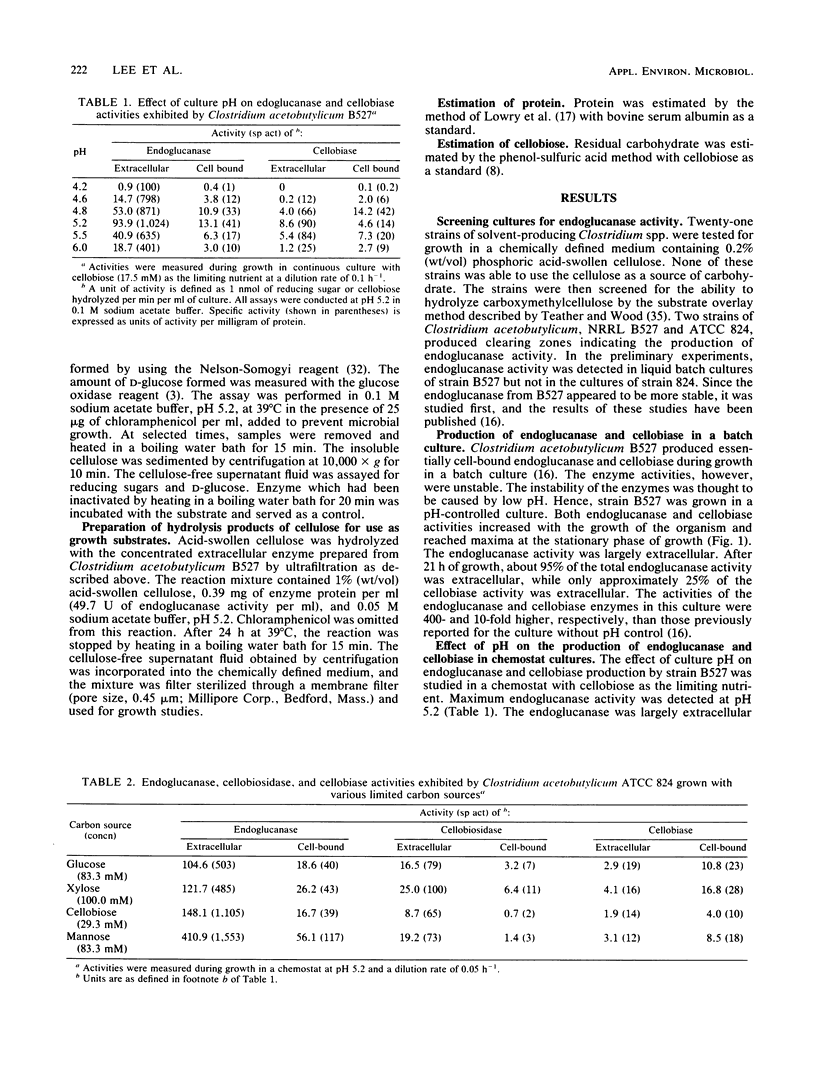
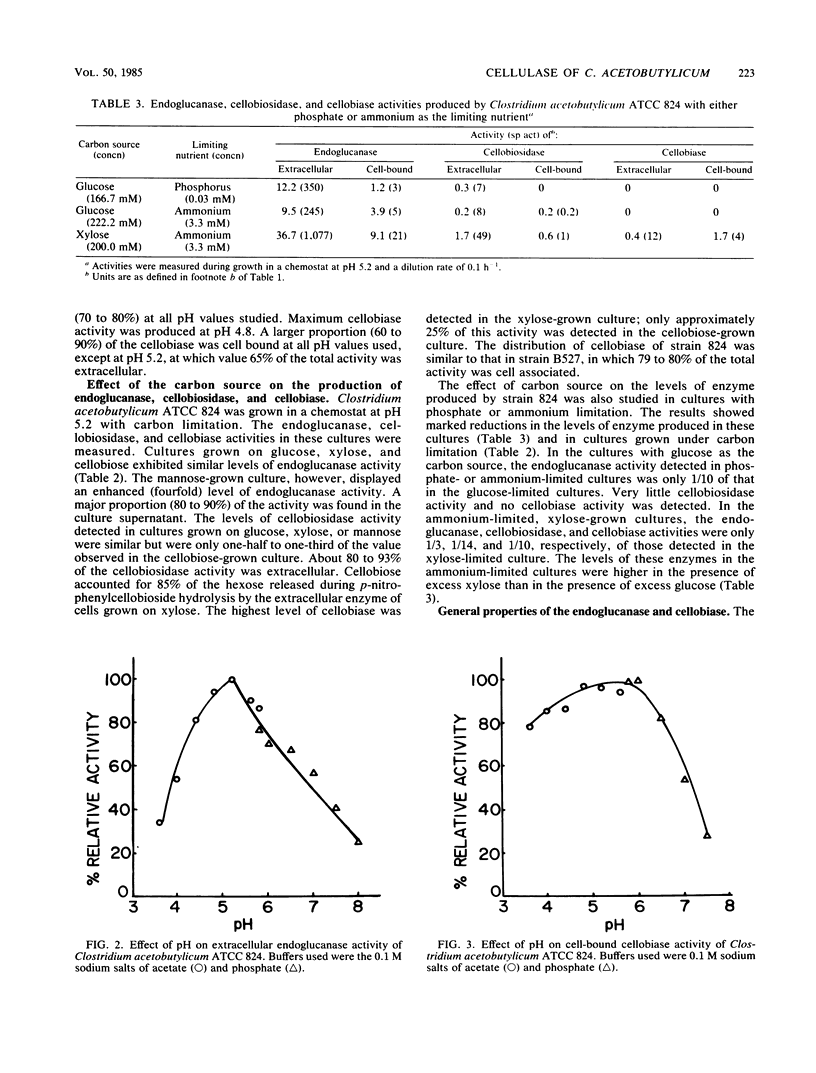
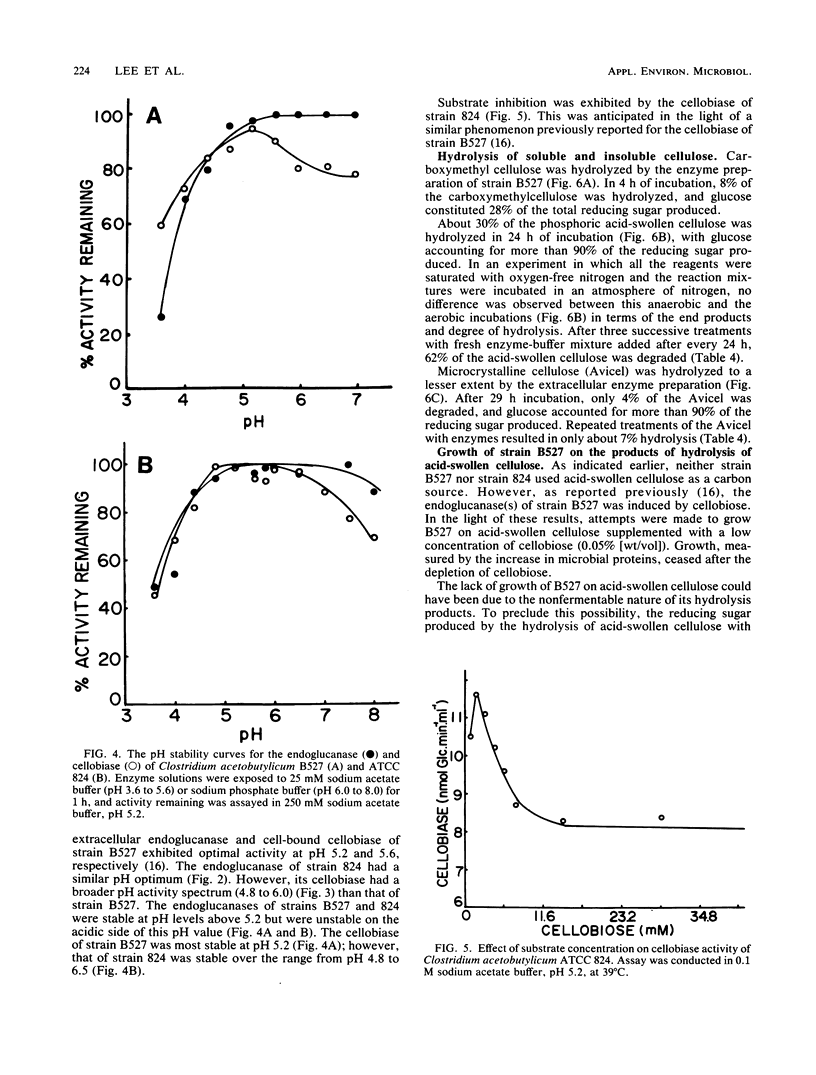
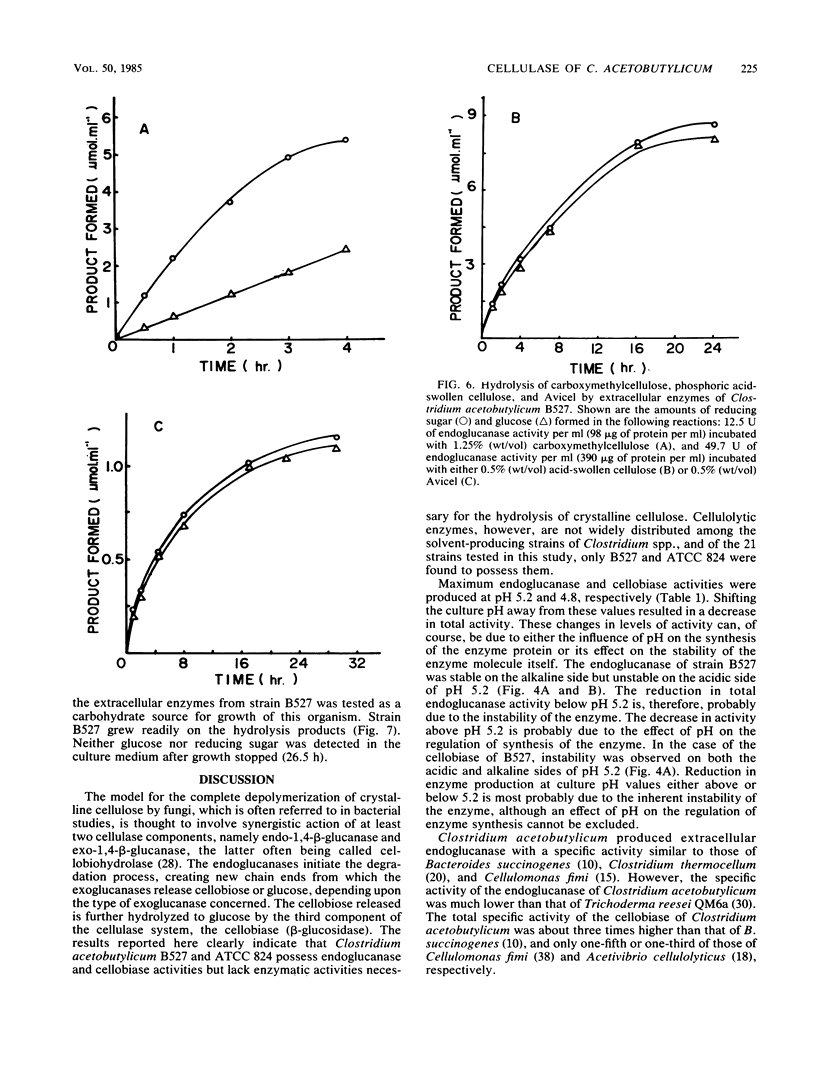
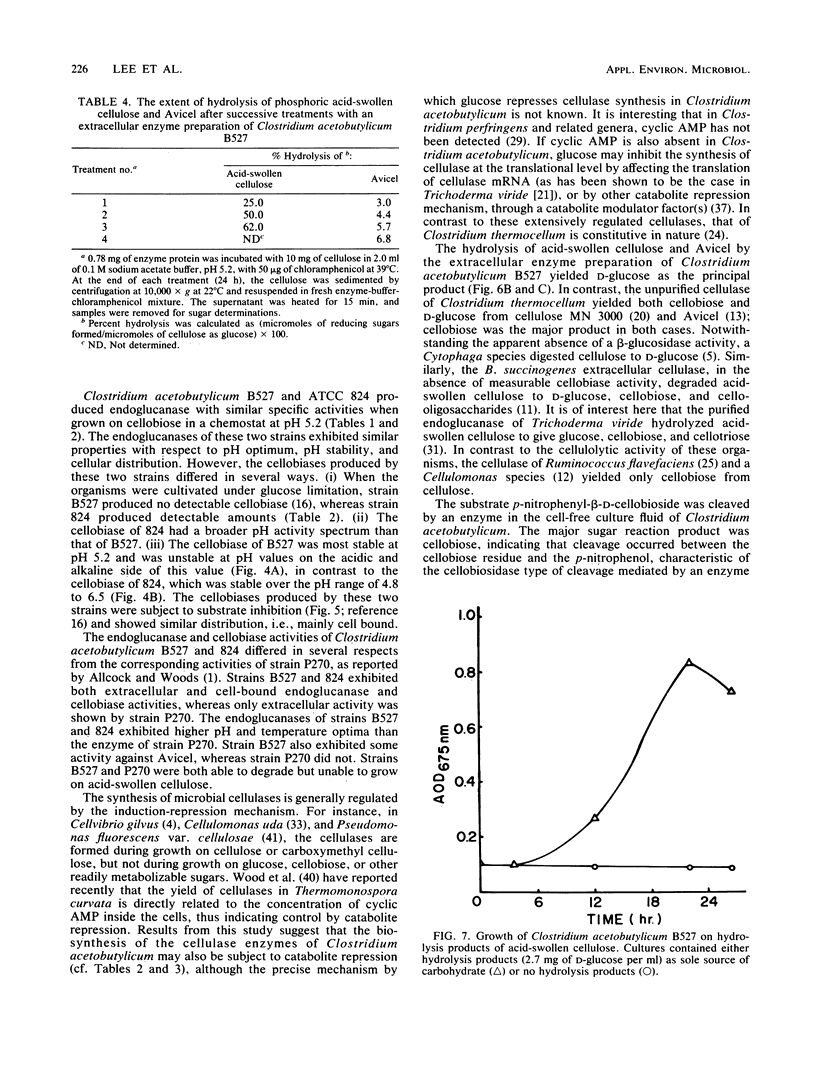
Clostridium acetobutylicum NRRL B527 and ATCC 824 exhibited extracellular and cell-bound endoglucanase and cellobiase activities during growth in a chemically defined medium with cellobiose as the sole source of carbohydrate. For both strains, the endoglucanase was found to be mainly extracellular (70 to 90%) during growth in continuous or batch cultures with the pH maintained at 5.2, whereas the cellobiase was mainly cell associated (60 to 90%). During continuous cultivation of strain B527 with cellobiose as the limiting nutrient, maximum production of the endoglucanase and cellobiase occurred at pH values of 5.2 and 4.8, respectively. In the carbon-limited continuous cultures, strain 824 produced similar levels of endoglucanase, cellobiosidase, and cellobiase activities regardless of the carbon source used. However, in ammonium- or phosphate-limited cultures, with an excess of glucose, only 1/10 of the endoglucanase was produced, and neither cellobiosidase nor cellobiase activities were detectable. A crude extracellular enzyme preparation from strain B527 hydrolyzed carboxymethylcellulose and phosphoric acid-swollen cellulose readily and microcrystalline cellulose (A vicel) to a lesser extent. Glucose accounted for more than 90% of the reducing sugar produced by the hydrolysis of acid-swollen cellulose and Avicel. Strain B527 did not grow in medium with acid-swollen cellulose as the sole source of carbohydrate, although it grew readily on the products obtained by hydrolyzing the cellulose in vitro with a preparation of extracellular cellulase derived from the same organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcock E. R., Woods D. R. Carboxymethyl cellulase and cellobiase production by Clostridium acetobutylicum in an industrial fermentation medium. Appl Environ Microbiol. 1981 Feb;41(2):539–541. doi: 10.1128/aem.41.2.539-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuil C., Kushner D. J. Cellulase induction and the use of cellulose as a preferred growth substrate by Cellvibrio gilvus. Can J Microbiol. 1976 Dec;22(12):1776–1781. doi: 10.1139/m76-264. [DOI] [PubMed] [Google Scholar]
- Chang W. T., Thayer D. W. The cellulase system of a Cytophaga species. Can J Microbiol. 1977 Sep;23(9):1285–1292. doi: 10.1139/m77-192. [DOI] [PubMed] [Google Scholar]
- Deshpande M. V., Eriksson K. E., Pettersson L. G. An assay for selective determination of exo-1,4,-beta-glucanases in a mixture of cellulolytic enzymes. Anal Biochem. 1984 May 1;138(2):481–487. doi: 10.1016/0003-2697(84)90843-1. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Partial characterization of the extracellular carboxymethylcellulase activity produced by the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1983 May;29(5):504–517. doi: 10.1139/m83-080. [DOI] [PubMed] [Google Scholar]
- Han Y. W., Srinivasan V. R. Isolation and characterization of a cellulose-utilizing bacterium. Appl Microbiol. 1968 Aug;16(8):1140–1145. doi: 10.1128/am.16.8.1140-1145.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Sakajoh M., Halliwell G., Madia A., Demain A. L. Saccharification of Complex Cellulosic Substrates by the Cellulase System from Clostridium thermocellum. Appl Environ Microbiol. 1982 May;43(5):1125–1132. doi: 10.1128/aem.43.5.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ng T. K., Weimer T. K., Zeikus J. G. Cellulolytic and physiological properties of Clostridium thermocellum. Arch Microbiol. 1977 Jul 26;114(1):1–7. doi: 10.1007/BF00429622. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nisizawa K. Catabolite repression of cellulase formation in Trichoderma viride. J Biochem. 1972 Jun;71(6):999–1007. doi: 10.1093/oxfordjournals.jbchem.a129872. [DOI] [PubMed] [Google Scholar]
- Ohmiya K., Shimizu M., Taya M., Shimizu S. Purification and properties of cellobiosidase from Ruminococcus albus. J Bacteriol. 1982 Apr;150(1):407–409. doi: 10.1128/jb.150.1.407-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya K., Shirai M., Kurachi Y., Shimizu S. Isolation and properties of beta-glucosidase from Ruminococcus albus. J Bacteriol. 1985 Jan;161(1):432–434. doi: 10.1128/jb.161.1.432-434.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS D. The acetone-butanol fermentation. Prog Ind Microbiol. 1961;3:71–90. [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Setlow P., Sacks L. E. Cyclic AMP is not detectable in Clostridium perfringens. Can J Microbiol. 1983 Sep;29(9):1228–1230. doi: 10.1139/m83-189. [DOI] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Enzymic activities of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):133–146. doi: 10.1016/0005-2744(78)90016-5. [DOI] [PubMed] [Google Scholar]
- Teather R. M. Maintenance of Laboratory strains of obligately anaerobic rumen bacteria. Appl Environ Microbiol. 1982 Aug;44(2):499–501. doi: 10.1128/aem.44.2.499-501.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M. The cellulase of Fusarium solani. Purification and specificity of the -(1-4)-glucanase and the -D-glucosidase components. Biochem J. 1971 Feb;121(3):353–362. doi: 10.1042/bj1210353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. E., Neubauer D. G., Stutzenberger F. J. Cyclic AMP levels during induction and repression of cellulase biosynthesis in Thermomonospora curvata. J Bacteriol. 1984 Dec;160(3):1047–1054. doi: 10.1128/jb.160.3.1047-1054.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Suzuki H., Hirotani M., Ozawa H., Nisizawa K. Effect of nature and supply of carbon sources on cellulase formation in Pseudomonas fluorescens var. cellulosa. J Biochem. 1970 Jan;67(1):9–18. doi: 10.1093/oxfordjournals.jbchem.a129238. [DOI] [PubMed] [Google Scholar]


