Abstract
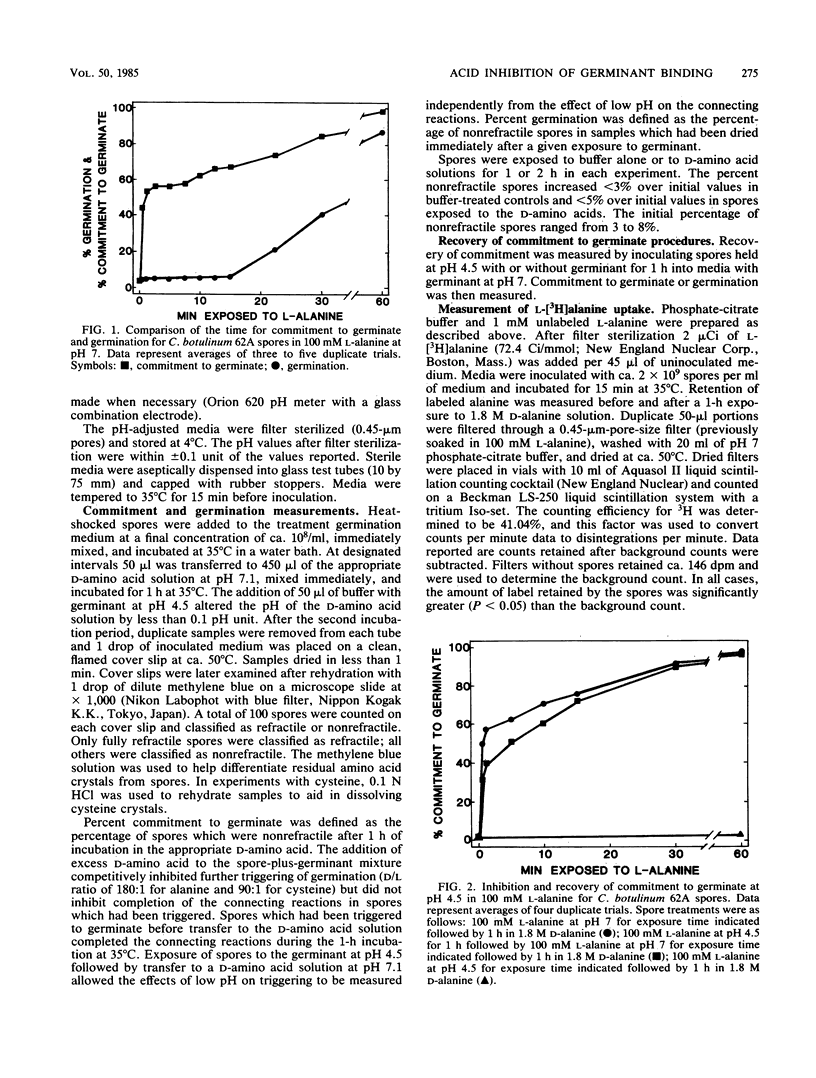
Commitment to germinate occurred in both Clostridium botulinum and Bacillus cereus spores during 0.5 min of exposure to 100 mM L-alanine or L-cysteine, measured by the inability of germination inhibitors (D form of amino acid) to inhibit germination. Spore germination at pH 4.5 was inhibited because the germinant did not bind to the trigger sites. C. botulinum spores exposed to 100 mM L-alanine or L-cysteine at pH 4.5 remained sensitive to D-amino acid inhibition at pH 7, indicating that no germinants had bound to the trigger site at pH 4.5. Inhibition of germinant binding at pH 4.5 was reversible but lagged in commitment to germinate upon transfer to pH 7. Spores sequentially exposed to pH 4.5 buffer and pH 7 buffer with the germinant also demonstrated a lag in commitment to germinate. The pH at which binding was inhibited was not significantly affected by composition of the buffer or by reduced germinant concentrations (10 mM). Nonspecific uptake of L-[3H]alanine by C. botulinum spores was not inhibited at pH 4.5. Inhibition of germinant binding in acidic environments appeared to be due to protonation of a functional group in or near the trigger site. This may represent a general mechanism for inhibition of spore germination in acidic environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. I. Characterization of glucose uptake. J Bacteriol. 1961 Nov;82:743–749. doi: 10.1128/jb.82.5.743-749.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Fitz-James P., Aronson A. I. Characterization of a Bacillus cereus protease mutant defective in an early stage of spore germination. J Bacteriol. 1978 Jan;133(1):336–344. doi: 10.1128/jb.133.1.336-344.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L. N., Johnston R. W., Kautter D. A., Howard J. W., Aunan W. J. Effect of nitrite and nitrate on toxin production by Clostridium botulinum and on nitrosamine formation in perishable canned comminuted cured meat. Appl Microbiol. 1973 Mar;25(3):357–362. doi: 10.1128/am.25.3.357-362.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foegeding P. M., Busta F. F. Hypochlorite injury of Clostridium botulinum spores alters germination responses. Appl Environ Microbiol. 1983 Apr;45(4):1360–1368. doi: 10.1128/aem.45.4.1360-1368.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALMANN M., KEYNAN A. Stages in germination of spores of Bacillus licheniformis. J Bacteriol. 1962 Dec;84:1187–1193. doi: 10.1128/jb.84.6.1187-1193.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRELL W. K., HALVORSON H. Studies on the role of L-alanine in the germination of spores of Bacillus terminalis. J Bacteriol. 1955 Mar;69(3):275–279. doi: 10.1128/jb.69.3.275-279.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson H. O. IV. : Dormancy, Germination, and Outgrowth. Bacteriol Rev. 1959 Dec;23(4):267–272. doi: 10.1128/br.23.4.267-272.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R. G., Reich R. R., Duncan C. L. Alteration in ultrastructure and germination of Clostridium perfringens type A spores following extraction of spore coats. Can J Microbiol. 1978 Dec;24(12):1526–1536. doi: 10.1139/m78-244. [DOI] [PubMed] [Google Scholar]
- Ogg J. E., Lee S. Y., Ogg B. J. A modified tube method for the cultivation and enumeration of anaerobic bacteria. Can J Microbiol. 1979 Sep;25(9):987–990. doi: 10.1139/m79-151. [DOI] [PubMed] [Google Scholar]
- Stewart G. S., Johnstone K., Hagelberg E., Ellar D. J. Commitment of bacterial spores to germinate. A measure of the trigger reaction. Biochem J. 1981 Jul 15;198(1):101–106. doi: 10.1042/bj1980101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973 Nov;116(2):797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Properties of Bacillus megaterium temperature-sensitive germination mutants. J Bacteriol. 1975 Jan;121(1):197–203. doi: 10.1128/jb.121.1.197-203.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgamott G. D., Durham N. N. Initiation of spore germination in Bacillus cereus: a proposed allosteric receptor. Can J Microbiol. 1971 Aug;17(8):1043–1048. doi: 10.1139/m71-165. [DOI] [PubMed] [Google Scholar]


