Abstract
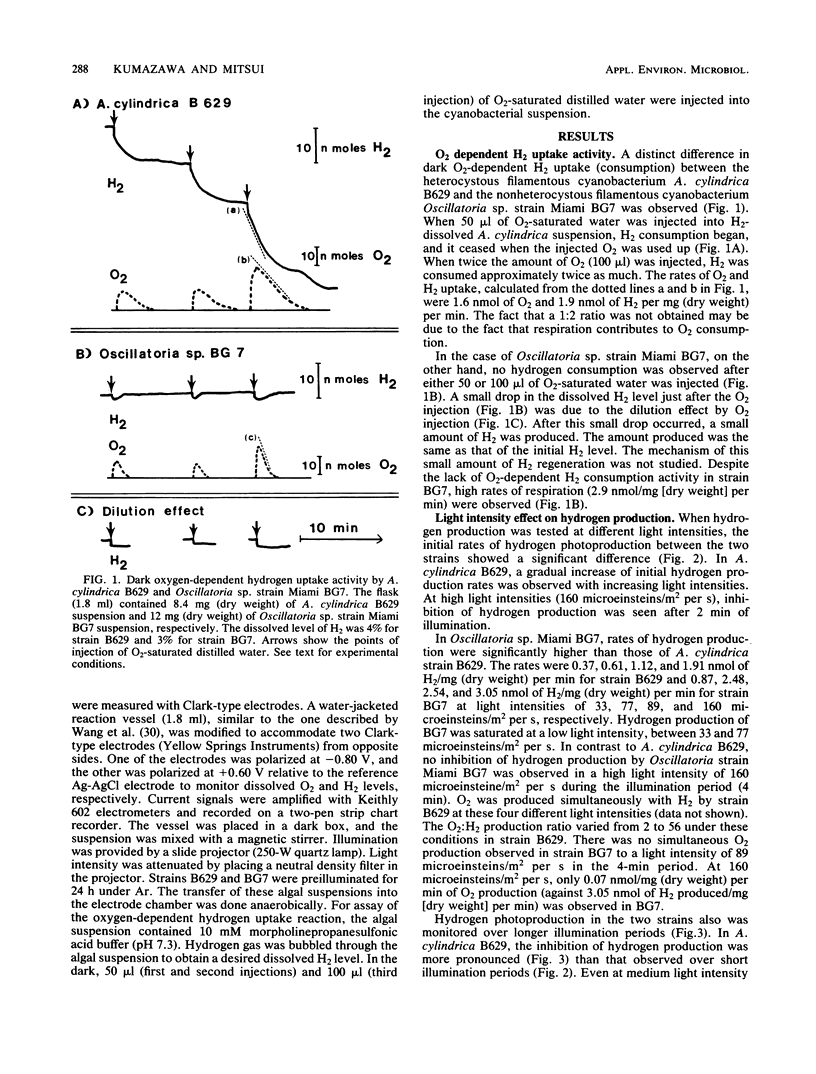
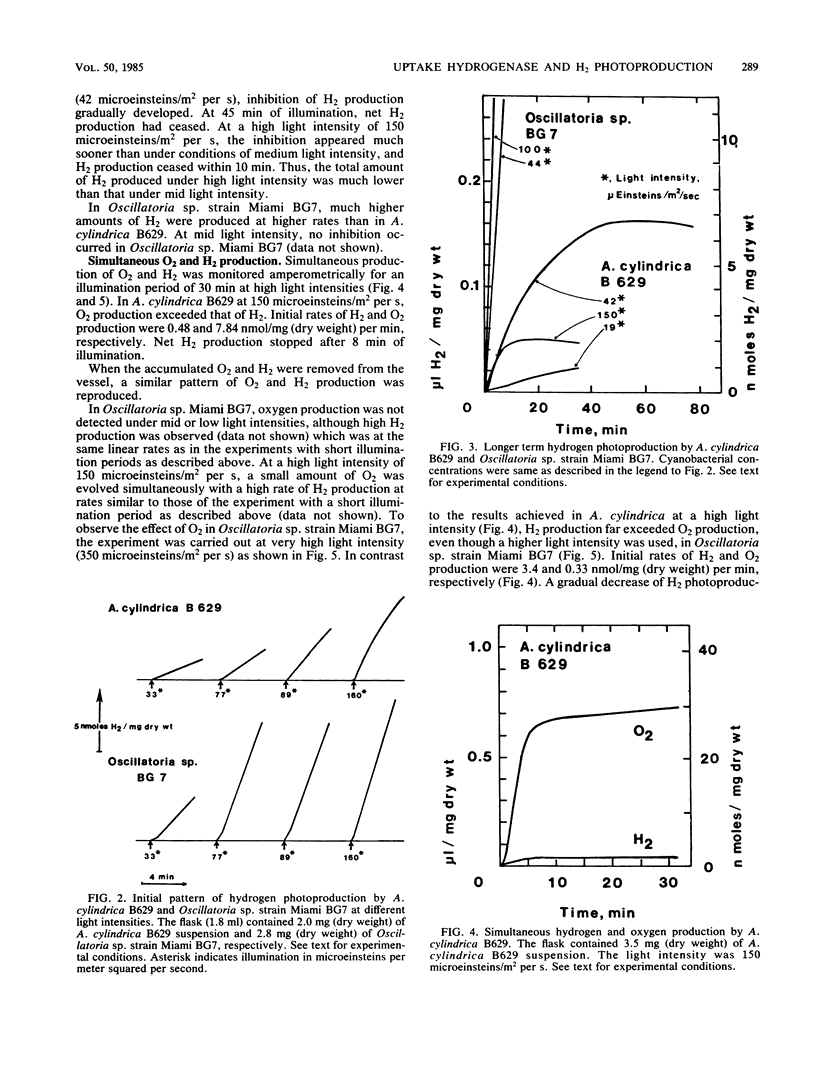
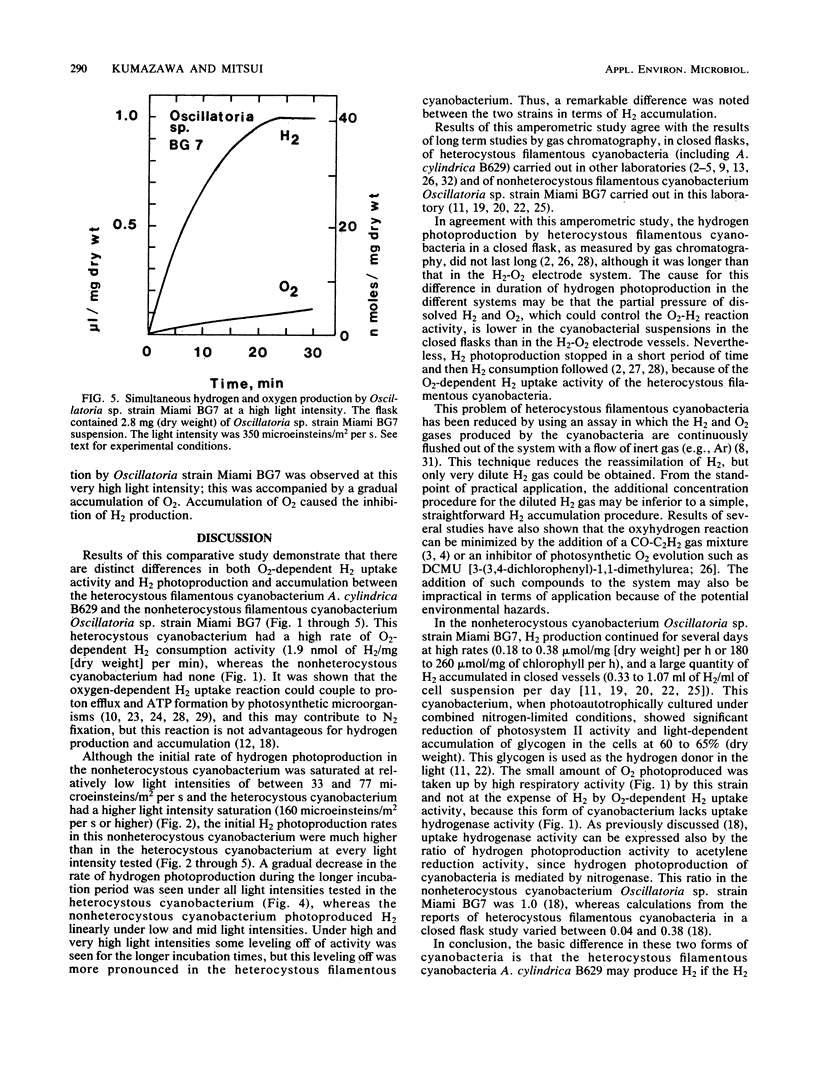
Heterocystous filamentous cyanobacterium Anabaena cylindrica B629 and nonheterocystous filamentous cyanobacterium Oscillatoria sp. strain Miami BG7 were cultured in media with N2 as the sole nitrogen source; and activities of oxygen-dependent hydrogen uptake, photohydrogen production, photooxygen evolution, and respiration were compared amperometrically under the same or similar experimental conditions for both strains. Distinct differences in these activities were observed in both strains. The rates of hydrogen photoproduction and hydrogen accumulation were significantly higher in Oscillatoria sp. strain BG7 than in A. cylindrica B629 at every light intensity tested. The major reason for the difference was attributable to the fact that the heterocystous cyanobacterium had a high rate of oxygen-dependent hydrogen consumption activity and the nonheterocystous cyanobacterium did not. The activity of oxygen photoevolution and respiration also contributed to the difference. Oscillatoria sp. strain BG7 had lower O2 evolution and higher respiration than did A. cylindrica B629. Thus, the effect of O2 on hydrogen photoproduction was minimized in Oscillatoria sp. strain BG7.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., Weare N. M. Hydrogen Evolution by Nitrogen-Fixing Anabaena cylindrica Cultures. Science. 1974 Apr 12;184(4133):174–175. doi: 10.1126/science.184.4133.174. [DOI] [PubMed] [Google Scholar]
- Bothe H., Distler E., Eisbrenner G. Hydrogen metabolism in blue-green algae. Biochimie. 1978;60(3):277–289. doi: 10.1016/s0300-9084(78)80824-4. [DOI] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Daday A., Platz R. A., Smith G. D. Anaerobic and aerobic hydrogen gas formation by the blue-green alga Anabaena cylindrica. Appl Environ Microbiol. 1977 Nov;34(5):478–483. doi: 10.1128/aem.34.5.478-483.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead A., Robinson R., Stewart W. D. Nitrogenase activity in extracts of heterocystous and non-heterocystous blue-green algae. Arch Mikrobiol. 1970;74(3):235–243. doi: 10.1007/BF00408884. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Timourian H., Ward R. L. Hydrogen production by Anabaena cylindrica: effects of varying ammonium and ferric ions, pH, and light. Appl Environ Microbiol. 1978 Apr;35(4):704–710. doi: 10.1128/aem.35.4.704-710.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Bishop N. I. Simultaneous measurement of oxygen and hydrogen exchange from the blue-green alga anabaena. Plant Physiol. 1976 Apr;57(4):659–665. doi: 10.1104/pp.57.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa S., Izawa S., Mitsui A. Proton efflux coupled to dark H2 oxidation in whole cells of a marine sulfur photosynthetic bacterium (Chromatium sp. strain Miami PBS1071). J Bacteriol. 1983 Apr;154(1):185–191. doi: 10.1128/jb.154.1.185-191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Smith G. D. Hydrogen metabolism by filamentous cyanobacteria. Arch Biochem Biophys. 1980 Nov;205(1):36–50. doi: 10.1016/0003-9861(80)90081-8. [DOI] [PubMed] [Google Scholar]
- Paul F., Colbeau A., Vignais P. M. Phosphorylation coupled to H2 oxidation by chromatophores from Rhodopseudomonas capsulata. FEBS Lett. 1979 Oct 1;106(1):29–33. doi: 10.1016/0014-5793(79)80688-2. [DOI] [PubMed] [Google Scholar]
- Phlips E. J., Mitsui A. Role of Light Intensity and Temperature in the Regulation of Hydrogen Photoproduction by the Marine Cyanobacterium Oscillatoria sp. Strain Miami BG7. Appl Environ Microbiol. 1983 Apr;45(4):1212–1220. doi: 10.1128/aem.45.4.1212-1220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D. Some aspects of structure and function in N2-fixing cyanobacteria. Annu Rev Microbiol. 1980;34:497–536. doi: 10.1146/annurev.mi.34.100180.002433. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. An inducible hydrogenase in cyanobacteria enhances n2 fixation. FEBS Lett. 1977;78(1):49–52. doi: 10.1016/0014-5793(77)80270-6. [DOI] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J. C., Benemann J. R. Hydrogen production by nitrogen-starved cultures of Anabaena cylindrica. Appl Environ Microbiol. 1977 Jan;33(1):123–131. doi: 10.1128/aem.33.1.123-131.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


