Abstract
DNA can be damaged by various intracellular and environmental alkylating agents to produce alkylation base lesions. These base damages, if not repaired promptly, may cause genetic changes that lead to diseases such as cancer. Recently, it was discovered that some of the alkylation DNA base damage can be directly removed by a family of proteins called the AlkB proteins that utilize a mononuclear non-heme iron(II) and α-ketoglutarate as cofactor and cosubstrate. These proteins activate dioxygen and perform an unprecedented oxidative deal-kylation of the alkyl adducts on DNA heteroatoms. This review summarizes the discovery of this activity and the recent research advances in studying this unique DNA repair pathway. The focus is placed on the chemical mechanism and function of these proteins.
Keywords: DNA repair, AlkB, ABH, Direct repair, Mononuclear non-heme iron, Dioxygenase, Oxidative dealkylation, Demethylation
1. Direct repair of alkylation DNA damage
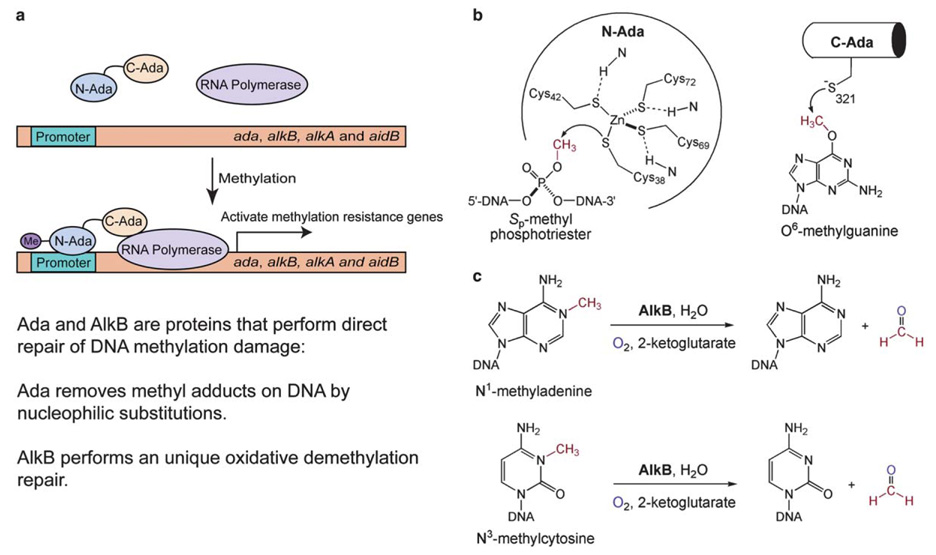
Cellular DNA is subject to nonenzymatic alkylation (methylation) by environmental or chemical mutagens, resulting in adducts that are toxic and mutagenic [1–6]. Organisms have evolved a variety of mechanisms to repair these mutagenic damages. Escherichia coli (E. coli) responds to this threat by activating an adaptive responsive pathway, mediated by the E. coli Ada protein [7]. Upon receiving a methyl group from the damaged DNA, Ada turns into a transcriptional activator and activates its own expression and that of the alkB gene and two other genes, alkA and aidB (Fig. 1(a)) [4,7,8]. The upregulated Ada is a bifunctional protein, which uses a N-terminal Cys38 residue to remove a methyl group fromSp-methyl-phosphotriester and a C-terminal Cys321 residue to remove a methyl adduct from O6-methylguanaine (Fig. 1(b)) [9–11]. Both repair functions are irreversible and represent rare examples of direct repair of DNA damage. AlkA is a glycosylase that cleaves methylated bases from DNA and performs the first step of the well-known base excision repair of base lesions [12–14]. The precise function of AidB is still unknown. The function of the last member of this adaptive response, AlkB, also remained unknown until recently despite extensive research efforts.
Fig. 1.
Schematic representations of the direct repair of methylation DNA damage in E. coli by proteins in the ada operon. (a) E. coli Ada is a transcriptional activator that senses methylation challenge and activates its own expression and three other genes, alkB, alkA and aidB. AlkA is a glycosylase that cleaves methylated bases from DNA. The function of AidB is still unknown. Both Ada and AlkB perform direct repair of methylation (alkylation) DNA damage. (b) Ada uses Cys residues to nucleophilically remove methyl groups from DNA backbone and bases. (c) AlkB catalyzes an oxidative dealkylation repair of DNA base lesions.
2. E. coli AlkB
Since the first genetic study in 1983 isolating the E. coli mutant with specific sensitivity to the alkylating agent methylmethane sulfonate, MMS [15], the activity of AlkB was undisclosed for almost 20 years. The research during this time included the finding of the resistance of AlkB to the treatment of the SN2 type alkylating agents [15,16], which suggested its involvement in protecting bacteria from the lethal effects of alkylation damage [15–18]. Results from a sequence alignment study of protein fold and sequence homology on AlkB [19] placed AlkB into the α-ketoglutarate (αKG)- and FeII-dependent dioxygenase family, a group of proteins that utilize FeII to activate dioxygen and perform oxidation of various substrates.
The FeII/αKG-dependent dioxygenase superfamily is widespread from bacteria to humans [19] and is the largest known non-heme iron protein family [20–23]. It represents a wide diversity of enzymes that catalyze the hydroxylation of unactivated C–H groups of a variety of substrates by coupling reductive activation of dioxygen with a decarbox-ylation of αKG, the co-substrate, to succinate. In the event of oxidation one of the oxygen atoms from O2 is incorporated into the succinate and the other as a hydroxyl in the product [24]. This group of enzymes is known for their importance in many environmental, pharmacological, and medical fields, and many of these reactions have not been successfully performed in the laboratory, especially ones involving the functionalization of inert C–H bonds. The reaction utilizes the mononuclear non-heme iron center, which is coordinated by a conserved His2/Asp or the less abundant His2/Glu motif to which dioxygen and αKG can also bind. Besides this rather simple, yet important motif, the members of this family have little sequence homology.
How can a DNA repair protein belong to an iron-containing dioxygenase family? DNA alkylation occurs due to reactions involving both SN1 and SN2 alkylating agents [25], and the susceptibility of each potential alkylation site on the bases or backbone varies on these reagents [2,6,25,26]. AlkB is responsible for repairing the damage caused by the SN2 type methylating agents such as MMS and methyl halides. These can react with single-stranded DNA (ssDNA) to generate large portions of N1-methyladenine (1-meA) and N3-methylcytosine (3-meC) [2,6,25,26] and they are the main methylation lesions formed [27–29]. These methyl adducts could be removed by two chemical mechanisms: (i) a direct displacement of the methyl group by a nucleophilic Cys residue or an activated water, which is similar to the mechanism used by the Ada proteins; or (ii) oxidation of the methyl group to give a hydroxyl intermediate, which decomposes in water to afford the repaired base and formaldehyde. In 2002, two groups simultaneously confirmed the second mechanism. They detected the formation of formaldehyde and showed that the AlkB protein utilizes a previously unknown oxidative demethylation pathway to repair 1-meA and 3-meC DNA damage (Fig. 1(c)) [30,31]. Thus, in the ada operon both Ada and AlkB use the direct repair pathways to remove methylation DNA damage. However, completely different mechanisms are adopted by the two proteins; Ada only performs stoichiometric repair, whereas AlkB catalyzes the oxidative removal of the methyl adducts (Fig. 1).
The N1-adenine and N3-cytosine positions are normally involved in hydrogen bonding and inaccessible in dsDNA. However, they are quite nucleophilic and vulnerable to attack by alkylating agents when exposed in ssDNA. Once alkylation has occurred on either of these two sites, the damaged bases can no longer form Watson–Crick base pairs. These non-coding lesions probably arise in vivo at DNA replication forks and transcription bubbles where they block the progression of DNA and RNA polymerases [32]. Thus, repair of these lesions is critical, as failure to repair these lesions in DNA leads to cell death in both E. coli and human cells [16].
3. AlkB homologs
Homologs of AlkB have been discovered based on sequence and fold similarity, and it is now known that the alkB gene is conserved from bacteria to human [19,33–35]. The homologs are not expressed equally in all human tissue types and may account for the discrepancies in function [36]. The first human homolog, now called ABH1, is 52% similar and 23% identical to the E. coli AlkB, but ABH1 along with ABH4, ABH6, and ABH7 did not show any activity on any of the known substrates for AlkB. Two other human homologs, ABH2 and ABH3 could complement the E. coli alkB mutant phenotype [37,38] and they, as well as two mouse homologs mAbh2 and mAbh3, were shown to function like AlkB [36]. The activities of ABH5 and ABH8 have not been evaluated so far. All homologs share the essential motifs and residues for enzymatic activity, consisting of a HXD motif, a single H, as well as a RXXXXXR motif where the X’s within the two arginines are alternating hydrophobic and polar residues (Fig. 2) [19,33–35]. The two histidines and the aspartic acid are proposed ligands to the active site FeII, whereas the first arginine is predicted to be involved in binding to the αKG [19,37] and these residues are highly conserved in the αKG-dependent oxygenases. This motif occurs as H131XD133…H187 for AlkB. These three ligands at the active site of AlkB were confirmed through chemical cross-linking studies, where each of these residues in AlkB, His131, Asp133, and His187, were mutated to a cysteine, and the mutant proteins were able to cross-link with DNA containing a modified thiol-tethered cytosine [39,40]. The cross-linking was inhibited by the addition of metal, as the cross-linking site was the FeII binding site [40].
Fig. 2.
Sequence alignment of the AlkB family of proteins. ABH1-7 are human homologs; ABH8* is the sequence from M. fascicularis; and mAbh2 and mAbh3 are mouse homologs. The conserved residues are highlighted in yellow (ligands for the active site iron) and blue (two conserved Arg residues). The RXXXXXR region is a characteristic of the AlkB sequence. The first Arg residue is thought to recognize α-ketoglutarate. The role of the second Arg residue is unclear.
4. The mononuclear iron center
The mononuclear FeII center of AlkB with bound αKG has been confirmed both with the overexpression and isolation of the native protein directly from E. coli [41] and by the addition of excess FeII and αKG to apo-AlkB [30–32,38,42–46]. Studies of the metal center has lead to an observation of an UV-vis band at 560 nm for the native protein [41]. This chromophore was assigned as the FeII to αKG charge transfer (MLCT) band, and is a spectroscopic characteristic of the FeII/αKG-dependent dioxygenases [47].
A slight change of the MLCT band was observed when an excess amount of DNA was added to the native AlkB. Further study of the protein and its DNA mixture by X-ray absorption spectroscopy provides evidence that the native AlkB binds DNA [41]. AlkB appears to have a five-coordinate FeII center in the absence of DNA, which becomes six-coordinate with the addition of ssDNA, suggesting a DNA-binding-induced geometry change of the mononuclear FeII center. Continued spectroscopic characterization of this iron center would be important to reveal properties of the Fe center in this family of novel proteins.
5. Repair mechanism
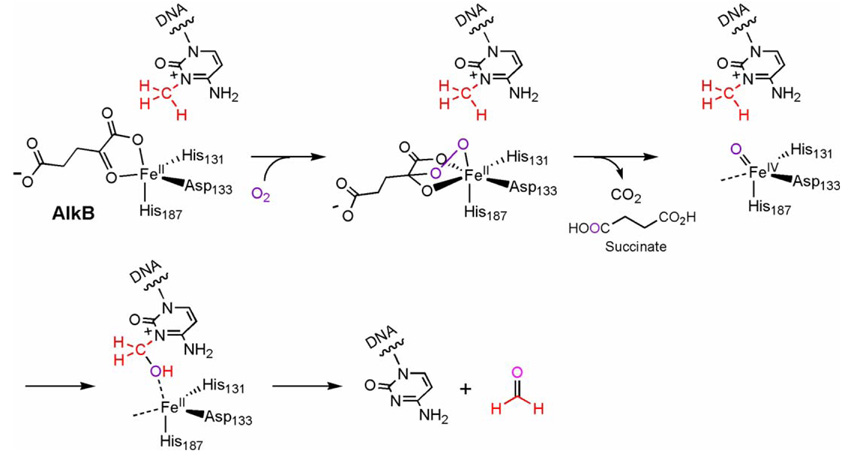
Addition of O2 saturated H2O to iron-loaded AlkB led to the development of a visible chromophore centered at 595 nm (ε590 960 M−1cm−1) [45]. This peak was attributed to the LMCT transition of OH-Trp coordinated to FeIII. The hydroxylation of the Trp side chain of AlkB, identified to be Trp178, suggests formation of an oxidative intermediate in AlkB [45]. This behavior is consistent with other members of the non-heme FeII/αKG-dependent family [48–50]. The mechanism for the reactions for these enzymes, including AlkB, is proposed to use an FeIV=O intermediate as the reactive oxidant. The proposed first step involves a reaction between the active site FeII and O2 to give a superoxo anion bound to FeIII. Subsequently, the nucleophilic superoxide attacks at the α-keto carbon of an iron-bound αKG to give a bridged peroxotype intermediate. This bridged intermediate then undergoes a concerted decarboxylation of αKG and a heterolytic cleavage of the O–O bond to form the high-valent FeIV=O intermediate [20–22]. This key active species is speculated to be used by enzymes in this family to hydroxylate C–H bonds of various substrates depending on the function of each protein. In AlkB this species hydroxylates the methyl adduct to give an unstable intermediate that decomposes in water to afford the final repaired product (Fig. 3). It should be noted that there have only been suggestions, as in the case of AlkB, and no concrete verification of the intermediates in the proposed mechanism, including the FeIV=O species, for most members of this family of enzymes.
Fig. 3.
A proposed oxidative repair mechanism of E. coli AlkB. A putative FeIV=O intermediate may form and hydroxylate the methyl lesion to give an intermediate that decomposes in water to afford the repaired base and formaldehyde.
The presence of the FeIV=O species has been revealed very recently for the enzyme Taurine/αKG dioxygenase, TauD. TauD catalyzes the hydroxylation of taurine to form sulfite, allowing the acquisition of sulfur from organic sulfonates for bacteria. TauD has been a preferred enzyme for study in this family due to its simplicity of substrate and its ease with which it can be prepared in large quantity, as well as the characterization of its crystal structure. The high spin FeIV complex of TauD was attained and characterized with kinetic and spectroscopic techniques, including absorption spectrum, Mossbauer, EXAFS and EPR experiments [51–53]. Model compounds also provide strong evidence for the existence of FeIV=O in proteins. Discrete mononuclear FeIV=O species have been synthesized and characterized recently [54–57]. These complexes serve as models for the intermediate species that may be generated in mononuclear non-heme iron-containing enzymes. In addition, ab initio calculations of non-heme intermediates support the likeliness of high spin FeIV=O species [58].
Enzymes in the FeII/αKG-dependent dioxygenase family can catalyze the reaction in which the substrate oxidation can be uncoupled from aKG decarboxylation, both with or without substrates [21,59]. This uncoupled turnover of αKG results in the decomposition of aKG into succinate and CO2 and usually leads to enzyme deactivation. Furthermore, this decomposition of aKG may account for the oxidation of the FeII center to form the inactive FeIII. This oxidation process can be reversed with addition of ascorbate [31,44] as it is considered to act as an electron source in the uncoupled reaction [21]. These uncoupled conversions possibly occur due to incorrect orientation of substrate in the active site, such that the substrates are never appropriately oxidized [59].
The uncoupled reaction, leading to irreversible modifications of AlkB [45], was demonstrated as αKG decomposition was found to be partially uncoupled from DNA repair, accounting for the observed stimulation of AlkB activity in the presence of ascorbate [31,44]. The uncoupled αKG turnover was also stimulated by binding of the small molecules 1-meA, 1-methyldeoxyadenosine (1-me(dA)), 3-meC and 3-methyldeoxycytidine (3-me(dC)), but they were not repaired by AlkB [44]. Unmethylated nucleosides did not stimulate αKG turnover, indicating that the presence of a modification in the substrate is important in initiating oxidation of αKG [44].
6. Substrates
AlkB repairs various modifications as shown by the survival of alkylated bacteriophage in an E. coli alkB mutant [32,37,46,60,61]. Mainly, AlkB is known for the repair of 1-meA and 3-meC. ABH2, ABH3, mAbh2, and mAbh3 repair these substrates, as well [36]. Ethyl and propyl DNA adducts at the N1-adenine and N3-cytosine also activate the repair function of AlkB [32,37,46,60,61]. The release of formaldehyde in the repair of methylated bases supports the mechanism of repair shown in Fig. 3, and studies have confirmed that 1-ethyladenine is repaired by AlkB to form adenine [37]. The C-1 C–H group rather than the C-2 C–H group of the ethyl adduct is oxidized, forming acetaldehyde as the released product along with the repaired base [37].
SN2 alkylating epoxides can cause hydroxyalkylated DNA lesions. For instance, ethylene oxide and propylene oxide can form 1-hydroxyalkyladenine and 3-hydroxyalkylcytosine. To create such lesions on DNA, iodoethanol, a reagent forming the same product as ethylene oxide, and propylene oxide was used to form hydroxyethyl and hydroxypropyl adducts at the N1-position of adenine. These lesions, too, were found to be repaired by AlkB [46]. Furthermore, methylations on the N3-position of thymine (3-meT) as well as the N1-position of guanine (1-meG) are also repaired by AlkB [32,42,61]. Human homologs ABH2 and ABH3 are able to demethylate 3-meT and 1-meG lesions in DNA oligonucleotides. A study on the mutagenicity, cytotoxicity and repair of 3-meT and 1-meG in wild type and alkB mutant strains of E. coli further indicates that these two lesions are indeed AlkB substrates [61].
3-meT is a minor product when ssDNA or dsDNA is treated with both SN2 (MMS and dimethylsulfate, DMS) and SN1 (methylnitrosourea, MNU) agents at neutral pH, constituting up to 0.8% of the total alkylated products. The yield of 3-meT is increased when DNA is treated with the SN1 agent under alkaline conditions or when free thy-midine is exposed to DMS at high pH [32]. 3me-T has good chemical stability, and the 3-position is normally involved in base pairing, thus, like 1-meA and 3-meC, is thought to be a non-coding lesion. Both 3-meT and 3-ethylthymine in synthetic polymers completely block DNA synthesis by E. coli DNA polymerase I or the Klenow fragment of this enzyme [32]. The physiological importance of these lesions and their repair remains to be unveiled.
Some conclusions may be drawn about the critical features of efficient repair by comparison of 3-meT and 1-meG with 1-meA and 3-meC. Repair of 3-meT by both AlkB and ABH3 was optimal at pH 6 (range of 5.5–6.5 for ABH3) but inefficient [32]. At physiological pH, 3-meT is more slowly repaired than the major lesion found in ssDNA, 3-meC. 1-meG is a major lesion generated when tRNA is treated with MeI but is also repaired slowly compared to 1-meA or 3-meC [42]. The 3-meC and 1-meA are drawn as positively charged in solution; however, they could also exist as the neutral 6-imino (for 1-meA) or 4-imino (for 3-meC) forms, respectively, after isomerization and release of one proton from the exocyclic amino groups [62,63]. The imino nitrogen could serve as a hydrogen bond acceptor or a ligand to the active site metal, which may facilitate recognition of these two substrates. The presence of either a positive charge or an imino group may be critical features for efficient recognition or repair by AlkB. These may also account for the differences in the optimal pH values for repair of these lesions (7.5–8 for 1-meA and 3-meC). Nonetheless, damage of 1-meA and 3-meC, in particular, are thought to be the physiological substrates of AlkB. The base lesions 3-meT and 1-meG, may be recognized differently by the enzymes [42] as they did not stimulate the uncoupled AlkB-mediated decarboxylation of αKG.
7. Oxidative repair of exocyclic DNA adducts
If a high valent iron-oxo species is generated in AlkB upon exposure to dioxygen, this species may oxidize other DNA adducts that block Watson–Crick base pairing. Exo-cyclic DNA adducts such as 1,N6-ethenoadenine (εA), 3,N4-ethenocytosine (εC), and 1,N2-ethenoguanine (εG) are produced when electrophilic vinyl chloride (VC) metabolites, chloroethylene oxide (CEO) or chloroacetaldehyde (CAA) react with adenine, cytosine, and guanine residues in DNA [28], respectively. The etheno bridges completely block normal Watson–Crick base pairing and are cytotoxic and potentially mutagenic [27]. These DNA adducts can arise endogeneously under oxidative stress in human cells, in particular when DNA is exposed to side products of lipid peroxidation [64–66]. The accumulation of these DNA lesions has been linked to aging and various diseases. It is known that the adaptive response pathway in E. coli renders the microbe resistant to induction of mutation by CAA [67,68], a hint that exocyclic DNA adducts are processed by the induced proteins.
Indeed two recent reports demonstrated that E. coli AlkB can repair εA and perhaps εC lesions in DNA [69,70]. Direct repair of εA in DNA by AlkB seems to be efficient both in vitro and in vivo. An epoxide intermediate was trapped and identified by MALDI-TOF mass spectrometry when a single-stranded 16 mer DNA containing a damaged base εA was used as the substrate [70]. This result supports a repair mechanism proposed in Fig. 4: the putative FeIV=O species can epoxidize the exocyclic double bond of the damaged base to give the epoxide, which is further hydrolyzed in water to afford the repaired base and glyoxal. Production of glyoxal was detected after the repair process in both reports [69,70]. Although turnovers could be observed within minutes for the repair of εA by E. coli AlkB [69], this protein is more active in repairing N1-methyladenine than εA [70]. The repair efficiency of εC is quite low [70], and its physiological relevance is unclear. ABH3, a human homolog of AlkB, exhibits the similar repair activity towards εA in DNA as E. coli AlkB [69]; however, the low reaction efficiency observed in vitro requires careful evaluation of this function in the future.
Fig. 4.
The AlkB proteins can directly repair exocyclic DNA adducts such as 1,N6-ethenoadenine. An epoxide intermediate was observed during the course of the repair, suggesting an oxidative repair mechanism.
The exocyclic DNA adducts were known to be repaired through base excision pathways in the past [71–76]. Why does E. coli or perhaps other organisms possess an additional mechanism to remove these lesions? One possibility is that the AlkB proteins are used mostly for fixing damage occurred on ssDNA and RNA. Systematic comparison of the repair activity of AlkB with glycosylases would help provide further insight and reveal biological significance of this discovery in the future.
8. Repair of RNA damage
DNA damage is not the only damaged substrate repaired by AlkB. The N1-position of adenine and N3-position of cytosine in most RNAs are exposed in a similar way as they are in ssDNA. In fact, 1-meA and 3-meC occur naturally in transfer RNA in both prokaryotes and eukaryotes [25,77]. Repair of these lesions in RNA is required for correct RNA folding to allow efficient and accurate translation. AlkB and its human homolog ABH3 were found to effectively repair 1-meA and 3-meC lesions on RNA, a potentially important defense function for cells against alkylation damage [38,77]. AlkB, ABH2 and ABH3 are all able to demethylate 3meT and 1-meG lesions introduced by chemical methylation on tRNA as well [32,42,61]. This unique feature of the AlkB proteins suggests that these proteins may also play important roles in viruses, where RNA encodes genetic information. It will be very interesting to study the AlkB homologs in different viruses.
9. A new paradigm?
The discovery of the unique oxidative dealkylation repair of DNA/RNA alkylation damage by the AlkB proteins opens a new paradigm in DNA repair research and bioinorganic chemistry. It adds an important new function to those of the non-heme mononuclear iron proteins that activate dioxygen and perform oxidation catalysis. Further studies to reveal spectroscopic properties and the mechanisms of the AlkB proteins would greatly facilitate our understanding of these enzymes. More importantly, the discovery has also identified a new DNA repair function in human cells that may have biological significance.
At least eight homologs of AlkB exist in the human genome based on sequence alignment [34]. The functions of two of them, ABH2 and ABH3, as well as two mouse homologs mAbh2 and mAbh3 have been characterized, although the physiological roles of these proteins and other homologs are unclear. These AlkB proteins can also mediate repair of RNA damage, which is quite unique. Despite the similarities of the metal binding residues and motifs, the characterized homologs do show different properties. Although the first human homolog ABH1 was initially thought to behave similarly to AlkB, the purified gene product showed no activity [37–39]. ABH2 and ABH3, together with the mouse homologs mAbh2 and mAbh3, all show repair activities for both 1-meA and 3-meC with the same cofactors as AlkB [36]. ABH2 and ABH3 require longer polynucleotides compared to AlkB as they have low or no activity with trimer substrates, whereas AlkB shows activity on trimers that is competitive with that of longer DNA [46]. ABH2 and ABH3, although optimized for pH and cofactor requirements, had only 0.7% and 2%, respectively, of AlkB’s activity when assayed with 1-meA in poly(dA) [46]. Furthermore, ABH2 prefers 1-meA while ABH3 prefers 3-meC [37,38]. ABH2 and mAbh2 prefer dsDNA while ABH3 and mAbh3 are more like AlkB; they prefer ssDNA and even RNA [32,36,38,39,43]. These differences in the homologs could certainly be due to their different sub-cellular localizations in human cells, or these proteins may have unique cellular roles that await to be elucidated. A future focus will certainly be to elucidate the functions and physiological roles of these human homologs.
Alkylation chemotherapies are widely used in clinics to treat various tumors [2,78–83]. The presence of DNA deal-kylation repair activity of the human homolog of C-Ada has been shown to confer resistance to some of these therapies. Whether the AlkB homologs play similar roles in the resistance is still unclear and will surely be scrutinized in the future. Perhaps inhibitors for the human homologs of AlkB could be developed to enhance the therapeutic effects of the alkylation anticancer reagents.
The function of AlkB also reveals a novel mechanism for dealkylation of DNA damage. In principle, the same mechanism can be utilized to repair other alkylation damage on heteroatoms of DNA as those shown in Fig. 5. The AlkB homologs in viruses or in human cells may use the oxidative dealkylation mechanism to remove these other alkylation adducts. It is also possible that alkylated/methylated protein residues can be dealkylated through the similar pathway catalyzed by non-heme iron proteins. The discovery of the function of AlkB may only be a beginning of uncovering a new type of dealkylation/demethylation pathway in biology.
Fig. 5.
DNA/RNA alkylation damages that are known or could be repaired by the AlkB type proteins.
Acknowledgement
We thank the National Institutes of Health (GM071440) for supporting this research.
References
- 1.Sedgwick B, Lindahl T. Oncogene. 2002;21:8886–8894. doi: 10.1038/sj.onc.1205998. [DOI] [PubMed] [Google Scholar]
- 2.Sedgwick B. Nature Rev. Mol. Cell Biol. 2004;5:148–157. doi: 10.1038/nrm1312. [DOI] [PubMed] [Google Scholar]
- 3.Rydberg B, Lindahl T. EMBO. J. 1982;1:211–216. doi: 10.1002/j.1460-2075.1982.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindahl T, Sedgewick B, Sekiguchi M, Nakabeppu Y. Annu. Rev. Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- 5.Barrows LR, Magee PN. Carcinogenesis. 1982;3:349–351. doi: 10.1093/carcin/3.3.349. [DOI] [PubMed] [Google Scholar]
- 6.Drablos F, Feyzi E, Aas PA, Vaagbo CB, Kavli B, Bratlie MS, Pena-Diaz J, Otterlei M, Slupphaug G, Krokan HE. DNA Repair (Amst) 2004;3:1389–1407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Teo I, Sedgwick B, Kilpatrick MW, McCarthy TV, Lindahl T. Cell. 1986;45:315–324. doi: 10.1016/0092-8674(86)90396-x. [DOI] [PubMed] [Google Scholar]
- 8.Sakumi K, Sekiguchi M. J. Mol. Biol. 1989;205:373–385. doi: 10.1016/0022-2836(89)90348-3. [DOI] [PubMed] [Google Scholar]
- 9.Sedgwick B, Robins P, Totty N, Lindahl T. J. Biol. Chem. 1988;263:4430–4433. [PubMed] [Google Scholar]
- 10.He C, Hus J-C, Sun LJ, Zhou P, Norman DPG, Dötsch V, Gross JD, Lane WS, Wagner G, Verdine GL. Mol. Cell. 2005;20:117–129. doi: 10.1016/j.molcel.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Demple B, Sedgwick B, Robin P, Totty N, Waterfield MD, Lindahl T. Proc. Natl. Acad. Sci. USA. 1985;82:2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson K, Sahm J, Shenkar P, Strauss B. Mutat. Res. 1985;150:77–84. doi: 10.1016/0027-5107(85)90103-4. [DOI] [PubMed] [Google Scholar]
- 13.Labahn J, Scharer OD, Long A, Ezaz-Nikpay K, Verdine GL, Ellenberger TE. Cell. 1996;86:321–329. doi: 10.1016/s0092-8674(00)80103-8. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt MD, Allan JM, Lau AY, Ellenberger TE, Samson LD. BioEssays. 1999;21:668–676. doi: 10.1002/(SICI)1521-1878(199908)21:8<668::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Kataoka H, Yamamoto Y, Sekiguchi M. J. Bacteriol. 1983;153:1301–1307. doi: 10.1128/jb.153.3.1301-1307.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen BJ, Carroll P, Samson L. J. Bacteriol. 1994;176:6255–6261. doi: 10.1128/jb.176.20.6255-6261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka H, Sekiguchi M. Mol. Gen. Genet. 1985;198:263–269. doi: 10.1007/BF00383004. [DOI] [PubMed] [Google Scholar]
- 18.Kondo H, Nakabeppu Y, Kataoka H, Kuhara S, Kawabata S, Sekiguchi M. J. Biol. Chem. 1986;261:15772–15777. [PubMed] [Google Scholar]
- 19.Aravind L, Koonin EV. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-research0007. RESEARCH0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schofield CJ, Zhang Z. Curr. Opin. Struct. Biol. 1999;9:722–731. doi: 10.1016/s0959-440x(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 21.Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee S-K, Lehnert N, Neese F, Skulan AJ, Yang Y-S, Zhou J. Chem. Rev. 2000;100:235–349. doi: 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- 22.Que LJ. Nature Struct. Biol. 2000;7:182–184. doi: 10.1038/73270. [DOI] [PubMed] [Google Scholar]
- 23.Costas M, Mehn MP, Jensen MP, Que L., Jr Chem. Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 24.Welford RWD, Kirkpatrick JM, McNeill LA, Puri M, Oldham NJ, Schofield CJ. FEBS. Lett. 2005;579:5170–5174. doi: 10.1016/j.febslet.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Singer B, Grünberger D. Molecular Biology of Mutagens and Carcinogens. New york: Plenum Press; 1983. [Google Scholar]
- 26.Shooter KV, Howse R, Shah SA, Lawley PD. Biochem. J. 1974;137:303–312. doi: 10.1042/bj1370303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revich GG, Beattie KL. Carciogenesis. 1986;7:1569–1576. doi: 10.1093/carcin/7.9.1569. [DOI] [PubMed] [Google Scholar]
- 28.Barbin A, Bartsch H, Leconte P, Radman M. Nucleic Acids Res. 1981;9:375–387. doi: 10.1093/nar/9.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodell WJ, Singer B. Biochemistry. 1979;18:2860–2863. doi: 10.1021/bi00580a029. [DOI] [PubMed] [Google Scholar]
- 30.Falnes PO, Johansen RF, Seeberg E. Nature. 2002;419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 31.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 32.Koivisto P, Robins P, Lindahl T, Sedgwick B. J. Biol. Chem. 2004;279:40470–40474. doi: 10.1074/jbc.M407960200. [DOI] [PubMed] [Google Scholar]
- 33.Wei YF, Carter KC, Wang RP, Shell BK. Nucleic Acids Res. 1996;24:931–937. doi: 10.1093/nar/24.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurowski MA, Bhagwat AS, Papaj G, Bujnicki JM. BMC Genomics. 2003;4:48. doi: 10.1186/1471-2164-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombi D, Gomes SL. J. Bacteriol. 1997;179:3139–3145. doi: 10.1128/jb.179.10.3139-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D-H, Jin S-G, Cai S, Chen Y, Pfeifer GP, O’Connor TR. J. Biol. Chem. 2005;280:39448–39459. doi: 10.1074/jbc.M509881200. [DOI] [PubMed] [Google Scholar]
- 37.Duncan T, Trewick SC, Koivisto P, Bates PA, Lindahl T, Sedgwick B. Proc. Natl. Acad. Sci. USA. 2002;99:16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aas PA, Otterlei M, Falnes PO, Vagbo CB, Skorpen F, Akbari M, Sundheim O, Bjoras M, Slupphaug G, Seeberg E, Krokan HE. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 39.Mishina Y, Lee CH, He C. Nucleic Acids Res. 2004;32:1548–1554. doi: 10.1093/nar/gkh319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishina Y, He C. J. Am. Chem. Soc. 2003;125:8730–8731. doi: 10.1021/ja034636c. [DOI] [PubMed] [Google Scholar]
- 41.Mishina Y, Chen LX, He C. J. Am. Chem. Soc. 2004;126:16930–16936. doi: 10.1021/ja045066z. [DOI] [PubMed] [Google Scholar]
- 42.Falnes PO. Nucleic Acids Res. 2004;32:6260–6267. doi: 10.1093/nar/gkh964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falnes PO, Bjoras M, Aas PA, Sundheim O, Seeberg E. Nucleic Acids Res. 2004;32:3456–3461. doi: 10.1093/nar/gkh655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welford RW, Schlemminger I, McNeill LA, Hewitson KS, Schofield CJ. J. Biol. Chem. 2003;278:10157–10161. doi: 10.1074/jbc.M211058200. [DOI] [PubMed] [Google Scholar]
- 45.Henshaw TF, Feig M, Hausinger RP. J. Inorg. Biochem. 2004;98:856–861. doi: 10.1016/j.jinorgbio.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Koivisto P, Duncan T, Lindahl T, Sedgwick B. J. Biol. Chem. 2003;278:44348–44354. doi: 10.1074/jbc.M307361200. [DOI] [PubMed] [Google Scholar]
- 47.Hausinger RP. Crit. Rev. Biochem. Mol. Biol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 48.Ryle MJ, Koehntop KD, Liu A, Que L, Jr, Hausinger RP. Proc. Natl. Acad. Sci. USA. 2003;100:3790–3795. doi: 10.1073/pnas.0636740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryle MJ, Liu A, Muthukumaran RB, Ho RYN, Koehntop KD, McCracken J, Que L, Jr, Hausinger RP. Biochemistry. 2003;42:1854–1862. doi: 10.1021/bi026832m. [DOI] [PubMed] [Google Scholar]
- 50.Liu A, Ho RYN, Que L, Jr, Ryle MJ, Phinney BS, Hausinger RP. J. Am. Chem. Soc. 2001;123:5126–5127. doi: 10.1021/ja005879x. [DOI] [PubMed] [Google Scholar]
- 51.Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM., Jr J. Am. Chem. Soc. 2003;125:13008–13009. doi: 10.1021/ja037400h. [DOI] [PubMed] [Google Scholar]
- 52.Price JC, Barr EW, Tirupati B, Bollinger JM, Jr, Krebs C. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- 53.Riggs-Gelasco PJ, Price JC, Guyer RB, Brehm JH, Barr EW, Bollinger JM, Jr, Krebs C. J. Am. Chem. Soc. 2004;126:8108–8109. doi: 10.1021/ja048255q. [DOI] [PubMed] [Google Scholar]
- 54.Grapperhaus CA, Mienert B, Bill E, Weyhermuller T, Wieghardt K. Inorg. Chem. 2000;39:5306–5317. doi: 10.1021/ic0005238. [DOI] [PubMed] [Google Scholar]
- 55.Kaizer J, Costas M, Que L., Jr Angew. Chem. Int. Ed. Engl. 2003;42:3671–3673. doi: 10.1002/anie.200351694. [DOI] [PubMed] [Google Scholar]
- 56.Lim MH, Rohde JU, Stubna A, Bukowski MR, Costas M, Ho RY, Munck E, Nam W, Que L., Jr Proc. Natl. Acad. Sci. USA. 2003;100:3665–3670. doi: 10.1073/pnas.0636830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohde JU, In JH, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Munck E, Nam W, Que L., Jr Science. 2003;299:1037–1039. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh A, Tangen E, Ryeng H, Taylor PR. Eur. J. Inorg. Chem. 2004;2004:4555–4560. [Google Scholar]
- 59.Lee H-J, Lloyd MD, Harlos K, Clifton IJ, Baldwin JE, Schofield CJ. J. Mol. Biol. 2001;308:937–948. doi: 10.1006/jmbi.2001.4649. [DOI] [PubMed] [Google Scholar]
- 60.Dinglay S, Gold B, Sedgwick B. Mutat. Res. 1998;407:109–116. doi: 10.1016/s0921-8777(97)00065-7. [DOI] [PubMed] [Google Scholar]
- 61.Delaney JC, Essigmann JM. Proc. Natl. Acad. Sci. USA. 2004;101:14051–14056. doi: 10.1073/pnas.0403489101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veldhuyzen WF, Shallop AJ, Jones RA, Rokita SE. J. Am. Chem. Soc. 2001;123:11126–11132. doi: 10.1021/ja011686d. [DOI] [PubMed] [Google Scholar]
- 63.Roitzsch M, Lippert B. J. Am. Chem. Soc. 2004;126:2421. doi: 10.1021/ja038834f. [DOI] [PubMed] [Google Scholar]
- 64.Pollack M, Oe T, Lee SH, Silva Elipe MV, Arison BH, Blair IA. Chem. Res. Toxicol. 2003;16:893–900. doi: 10.1021/tx030009p. [DOI] [PubMed] [Google Scholar]
- 65.Nair J, Barbin A, Guichard Y, Bartsch H. Carcinogenesis. 1995;16:613–617. doi: 10.1093/carcin/16.3.613. [DOI] [PubMed] [Google Scholar]
- 66.Chung FL, Chen HJ, Nath RG. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 67.Mroczkowska MM, Kolasa IK, Kusmierek JT. Mutagenesis. 1993;8:341–348. doi: 10.1093/mutage/8.4.341. [DOI] [PubMed] [Google Scholar]
- 68.Borys E, Mroczkowska-Slupska MM, Kusmierek JT. Mutagenesis. 1994;9:407–410. doi: 10.1093/mutage/9.5.407. [DOI] [PubMed] [Google Scholar]
- 69.Mishina Y, Yang C-G, He C. J. Am. Chem. Soc. 2005;127:14594–14595. doi: 10.1021/ja055957m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delaney JC, Smeester L, Wong C, Frick LE, Taghizadeh K, Wishnok JS, Drennan CL, Samson LD, Essigmann JM. Nat. Struct. Mol. Biol. 2005;12:855–860. doi: 10.1038/nsmb996. [DOI] [PubMed] [Google Scholar]
- 71.Asaeda A, Ide H, Asagoshi K, Matsuyama S, Tano K, Murakami A, Takamori Y, Kubo K. Biochemistry. 2000;39:1959–1965. doi: 10.1021/bi9917075. [DOI] [PubMed] [Google Scholar]
- 72.O’Connor TR, Laval J. Biochem. Biophys. Res. Commun. 1991;176:1170–1177. doi: 10.1016/0006-291x(91)90408-y. [DOI] [PubMed] [Google Scholar]
- 73.Lau AY, Wyatt MD, Glassner BJ, Samson LD, Ellenberger T. Proc. Natl. Acad. Sci. USA. 2000;97:13573–13578. doi: 10.1073/pnas.97.25.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dosanjh MK, Roy R, Mitra S, Singer B. Biochemistry. 1994;33:1624–1628. doi: 10.1021/bi00173a002. [DOI] [PubMed] [Google Scholar]
- 75.Roy R, Kennel SJ, Mitra S. Carcinogenesis. 1996;17:2177–2182. doi: 10.1093/carcin/17.10.2177. [DOI] [PubMed] [Google Scholar]
- 76.Saparbaev M, Laval J. Proc. Natl. Acad. Sci. USA. 1998;95:8508–8513. doi: 10.1073/pnas.95.15.8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ougland R, Zhang CM, Liiv A, Johansen RF, Seeberg E, Hou YM, Remme J, Falnes PO. Mol. Cell. 2004;16:107–116. doi: 10.1016/j.molcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Vaughan P, Lindahl T, Sedgwick B. Mutat. Res. 1993;293:249–257. doi: 10.1016/0921-8777(93)90076-s. [DOI] [PubMed] [Google Scholar]
- 79.Taverna P, Sedgwick B. J. Bacteriol. 1996;178:5105–5111. doi: 10.1128/jb.178.17.5105-5111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldman R, Shields PG. J. Nutr. 2003;133:965S–973S. doi: 10.1093/jn/133.3.965S. [DOI] [PubMed] [Google Scholar]
- 81.Hecht SS. Mutat. Res. 1999;424:127–142. doi: 10.1016/s0027-5107(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 82.Hurley LH. Nature Rev. Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- 83.Rajski SR, Williams RM. Chem. Rev. 1998;98:2723–2795. doi: 10.1021/cr9800199. [DOI] [PubMed] [Google Scholar]