Abstract
We compared the metabolism of methanol and acetate when Methanosarcina barkeri was grown in the presence and absence of Desulfovibrio vulgaris. The sulfate reducer was not able to utilize methanol or acetate as the electron donor for energy metabolism in pure culture, but was able to grow in coculture. Pure cultures of M. barkeri produced up to 10 μmol of H2 per liter in the culture headspace during growth on acetate or methanol. In coculture with D. vulgaris, the gaseous H2 concentration was ≤2 μmol/liter. The fractions of 14CO2 produced from [14C]methanol and 2-[14C]acetate increased from 0.26 and 0.16, respectively, in pure culture to 0.59 and 0.33, respectively, in coculture. Under these conditions, approximately 42% of the available electron equivalents derived from methanol or acetate were transferred and were utilized by D. vulgaris to reduce approximately 33 μmol of sulfate per 100 μmol of substrate consumed. As a direct consequence, methane formation in cocultures was two-thirds that observed in pure cultures. The addition of 5.0 mM sodium molybdate or exogenous H2 decreased the effects of D. vulgaris on the metabolism of M. barkeri. An analysis of growth and carbon and electron flow patterns demonstrated that sulfate-dependent interspecies H2 transfer from M. barkeri to D. vulgaris resulted in less methane production, increased CO2 formation, and sulfide formation from substrates not directly utilized by the sulfate reducer as electron donors for energy metabolism and growth.
Full text
PDF

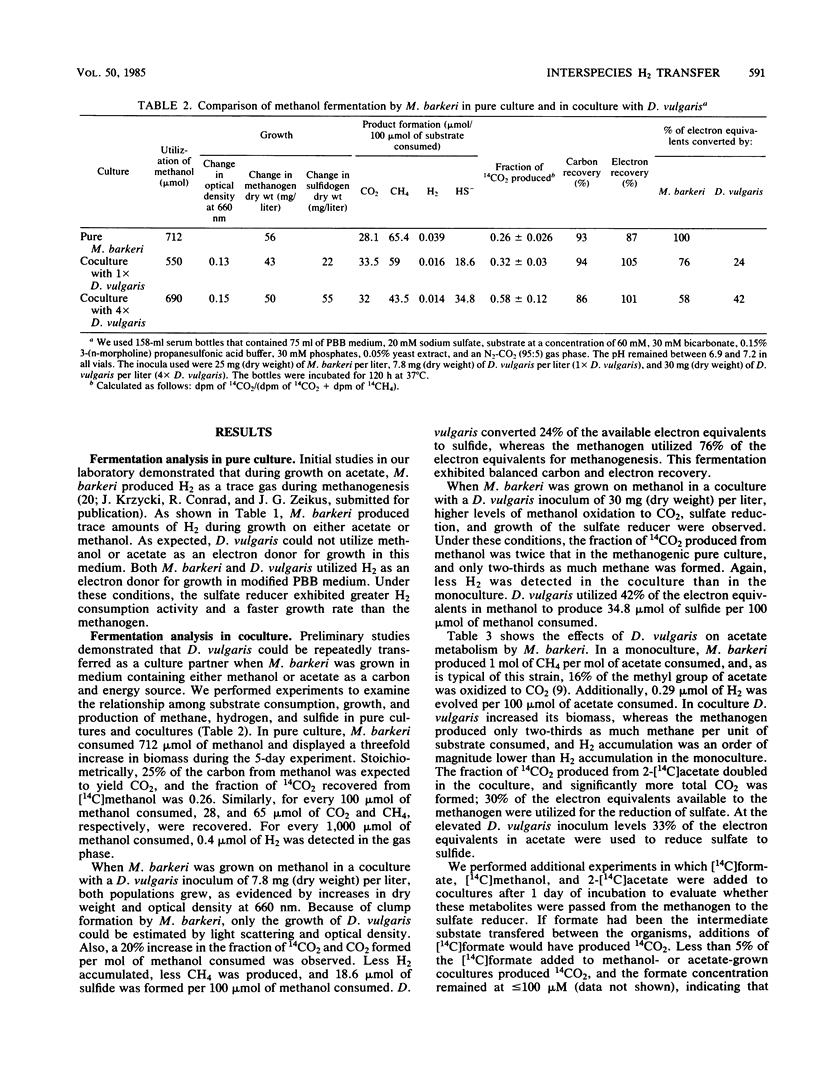
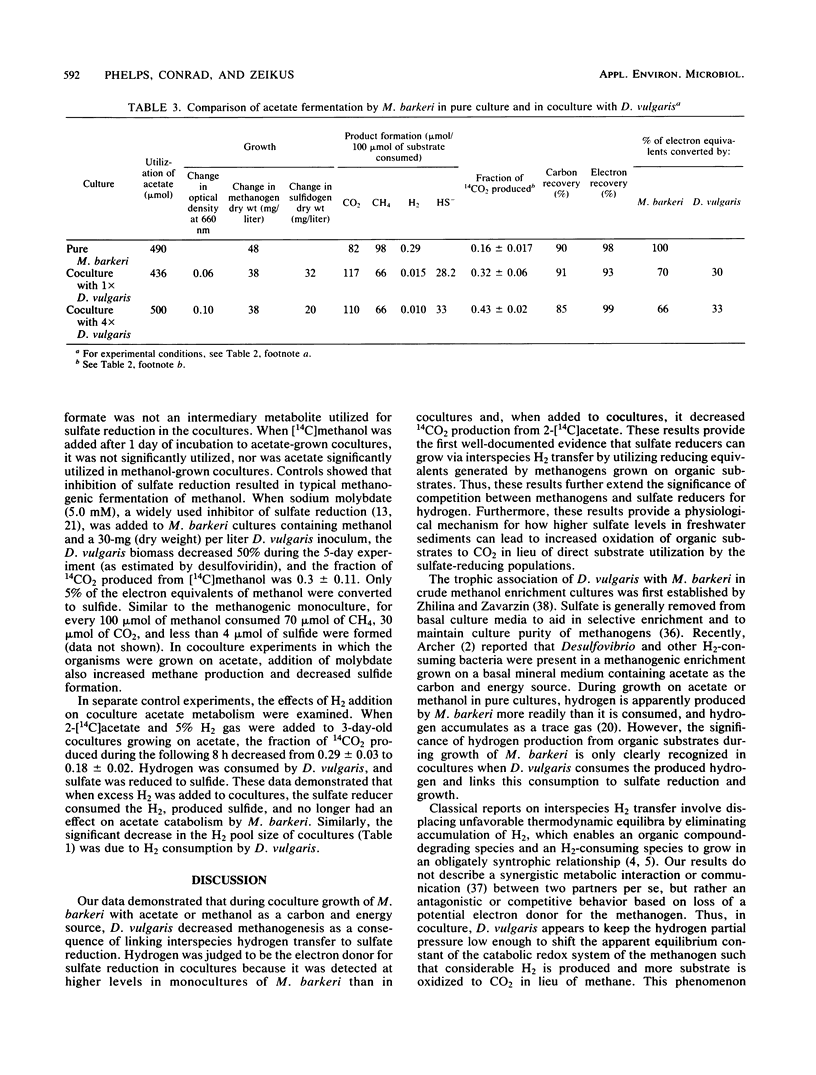


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram J. W., Nedwell D. B. Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch Microbiol. 1978 Apr 27;117(1):89–92. doi: 10.1007/BF00689356. [DOI] [PubMed] [Google Scholar]
- Archer D. B. Hydrogen-using bacteria in a methanogenic acetate enrichment culture. J Appl Bacteriol. 1984 Feb;56(1):125–129. doi: 10.1111/j.1365-2672.1984.tb04703.x. [DOI] [PubMed] [Google Scholar]
- Badziong W., Thauer R. K., Zeikus J. G. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol. 1978 Jan 23;116(1):41–49. doi: 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Campbell L. L., Reddy C. A., Crabill M. R. Growth of desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol. 1977 May;33(5):1162–1169. doi: 10.1128/aem.33.5.1162-1169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Iannotti E. L., Kafkewitz D., Wolin M. J., Bryant M. P. Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H 2 . J Bacteriol. 1973 Jun;114(3):1231–1240. doi: 10.1128/jb.114.3.1231-1240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerby R., Niemczura W., Zeikus J. G. Single-carbon catabolism in acetogens: analysis of carbon flow in Acetobacterium woodii and Butyribacterium methylotrophicum by fermentation and 13C nuclear magnetic resonance measurement. J Bacteriol. 1983 Sep;155(3):1208–1218. doi: 10.1128/jb.155.3.1208-1218.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzycki J. A., Wolkin R. H., Zeikus J. G. Comparison of unitrophic and mixotrophic substrate metabolism by acetate-adapted strain of Methanosarcina barkeri. J Bacteriol. 1982 Jan;149(1):247–254. doi: 10.1128/jb.149.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Dwyer D. F., Klug M. J. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol. 1982 Jun;43(6):1373–1379. doi: 10.1128/aem.43.6.1373-1379.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Ferry J. G. Production and Consumption of H(2) during Growth of Methanosarcina spp. on Acetate. Appl Environ Microbiol. 1985 Jan;49(1):247–249. doi: 10.1128/aem.49.1.247-249.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl Environ Microbiol. 1983 Jan;45(1):187–192. doi: 10.1128/aem.45.1.187-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton F. S., Conrad R., Zeikus J. G. Physiological function of hydrogen metabolism during growth of sulfidogenic bacteria on organic substrates. J Bacteriol. 1984 Sep;159(3):843–849. doi: 10.1128/jb.159.3.843-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L. H., Zeikus J. G. Metabolism of H2-CO2, methanol, and glucose by Butyribacterium methylotrophicum. J Bacteriol. 1983 Mar;153(3):1415–1423. doi: 10.1128/jb.153.3.1415-1423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. M., Wolkin R. H., Moench T. T., Morgan J. B., Zeikus J. G. Association of hydrogen metabolism with unitrophic or mixotrophic growth of Methanosarcina barkeri on carbon monoxide. J Bacteriol. 1984 Apr;158(1):373–375. doi: 10.1128/jb.158.1.373-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. A diagnostic reaction of Desulphovibrio desulphuricans. Nature. 1959 Feb 14;183(4659):481–482. doi: 10.1038/183481b0. [DOI] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P., Wolin M. J. Characteristics of S organism isolated from Methanobacillus omelianskii. J Bacteriol. 1972 Feb;109(2):539–545. doi: 10.1128/jb.109.2.539-545.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C. C., Linehan B., Wolin M. J. H2 production by Selenomonas ruminantium in the absence and presence of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):480–483. doi: 10.1128/am.29.4.480-483.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B., Stieb M. Fermentative degradation of polyethylene glycol by a strictly anaerobic, gram-negative, nonsporeforming bacterium, Pelobacter venetianus sp. nov. Appl Environ Microbiol. 1983 Jun;45(6):1905–1913. doi: 10.1128/aem.45.6.1905-1913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Nelson D. R., Klevickis S. C., Zeikus J. G. Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl Environ Microbiol. 1977 Feb;33(2):312–318. doi: 10.1128/aem.33.2.312-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Anaerobic metabolism of immediate methane precursors in Lake Mendota. Appl Environ Microbiol. 1979 Feb;37(2):244–253. doi: 10.1128/aem.37.2.244-253.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Microbial methanogenesis and acetate metabolism in a meromictic lake. Appl Environ Microbiol. 1979 Feb;37(2):213–221. doi: 10.1128/aem.37.2.213-221.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J. Metabolic interactions among intestinal microorganisms. Am J Clin Nutr. 1974 Nov;27(11):1320–1328. doi: 10.1093/ajcn/27.11.1320. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhilina T. N., Zavarzin G. A. Troficheskie sviazi metanosartsinoi i ee sputnikami. Mikrobiologiia. 1973 Mar-Apr;42(2):266–273. [PubMed] [Google Scholar]


