Abstract
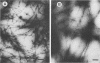
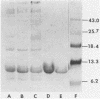
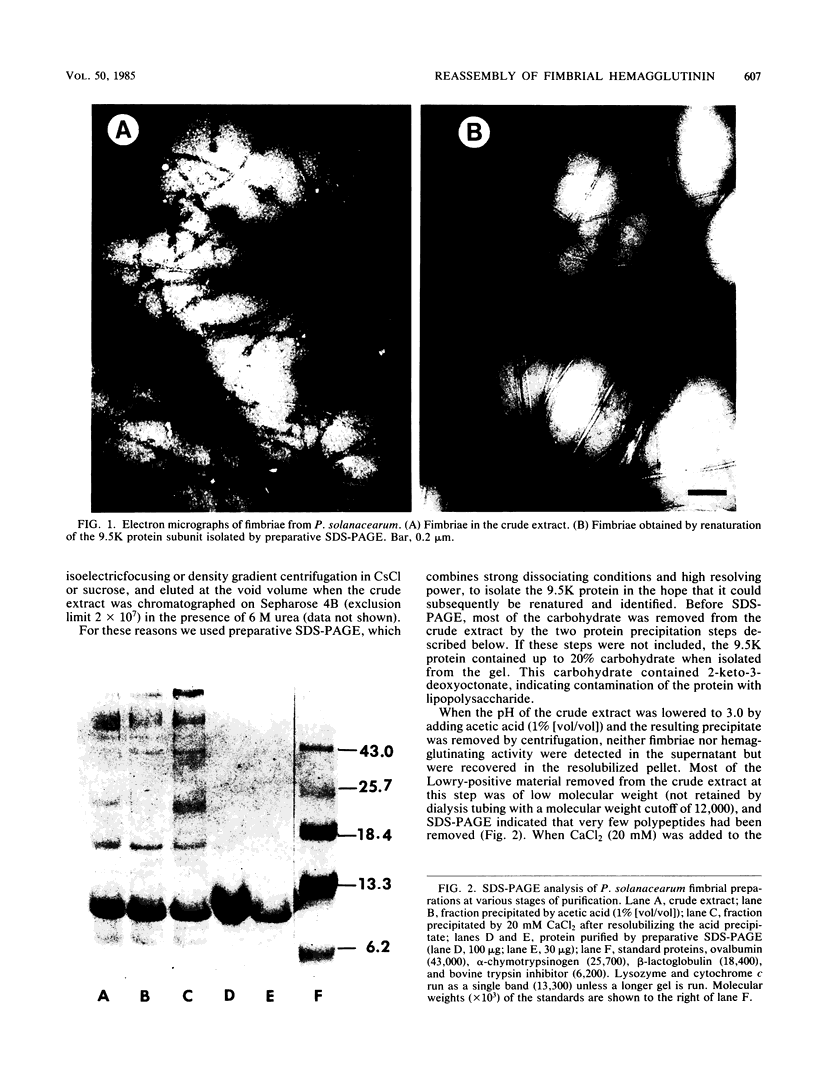
Distilled water homogenates of Pseudomonas solanacearum B1, a highly fimbriated strain, strongly agglutinated human group A erythrocytes. The fimbriae and hemagglutinating activity were precipitated from the crude extract with 1% acetic acid, redissolved at pH 10, and precipitated again with 20 mM CaCl2 at pH 6.9. Ca2+, Mg2+, and Zn2+ had similar ability to precipitate the fimbrial hemagglutinin, but Na+ and K+ were much less effective. The fimbrial protein in the precipitate was purified to homogeneity by preparative gel electrophoresis in sodium dodecyl sulfate. The major protein band was eluted, and sodium dodecyl sulfate was removed by chromatography on ion retardation resin (AG 11A8) in 6 M urea. After dialysis against 10 mM sodium acetate (pH 4.5) to remove the urea, the protein reassembled to yield long fibers. These fibers were identical to fimbriae in the crude extract in diameter (6 nm) and in their ability to cause hemagglutination. The purified fimbriae contained no carbohydrates and wee similar to other bacterial fimbriae in amino acid composition, with hydrophobic amino acids comprising 41.8% of the total.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. L., Berry R. W., Telser A. A sodium dodecyl sulfate--polyacrylamide gel electrophoresis system that separates peptides and proteins in the molecular weight range of 2500 to 90,000. Anal Biochem. 1983 Jul 15;132(2):365–375. doi: 10.1016/0003-2697(83)90022-2. [DOI] [PubMed] [Google Scholar]
- Duvick J. P., Sequeira L. Interaction of Pseudomonas solanacearum Lipopolysaccharide and Extracellular Polysaccharide with Agglutinin from Potato Tubers. Appl Environ Microbiol. 1984 Jul;48(1):192–198. doi: 10.1128/aem.48.1.192-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fuerst J. A., Hayward A. C. Surface appendages similar to fimbriae (pili) on pseudomonas species. J Gen Microbiol. 1969 Oct;58(2):227–237. doi: 10.1099/00221287-58-2-227. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Kapp O. H., Vinogradov S. N. Removal of sodium dodecyl sulfate from proteins. Anal Biochem. 1978 Nov;91(1):230–235. doi: 10.1016/0003-2697(78)90835-7. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kelman A., Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. J Gen Microbiol. 1973 May;76(1):177–188. doi: 10.1099/00221287-76-1-177. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIENER I. E. The photometric determination of the hemagglutinating activity of soyin and crude soybean extracts. Arch Biochem Biophys. 1955 Jan;54(1):223–231. doi: 10.1016/0003-9861(55)90025-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leach J. E., Cantrell M. A., Sequeira L. Hydroxyproline-rich bacterial agglutinin from potato : extraction, purification, and characterization. Plant Physiol. 1982 Nov;70(5):1353–1358. doi: 10.1104/pp.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y. C., Dunker A. K. Effects of metabolic inhibitors on the assembly of fd phage. Prog Clin Biol Res. 1981;64:467–474. [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Scraba D. G., Paranchych W. Formation of 9-nm filaments from pilin monomers obtained by octyl-glucoside dissociation of Pseudomonas aeruginosa pili. J Bacteriol. 1982 Sep;151(3):1508–1513. doi: 10.1128/jb.151.3.1508-1513.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]