Abstract
Gangliosides are expressed in the outer leaflet of the plasma membrane of the cells of all vertebrates and are particularly abundant in the nervous system. Ganglioside metabolism is closely associated with the pathology of Alzheimer's disease (AD). AD, the most common form of dementia, is a progressive degenerative disease of the brain characterized clinically by progressive loss of memory and cognitive function and eventually death. Neuropathologically, AD is characterized by amyloid deposits or “senile plaques,” which consist mainly of aggregated variants of amyloid β-protein (Aβ). Aβ undergoes a conformational transition from random coil to ordered structure rich in β-sheets, especially after addition of lipid vesicles containing GM1 ganglioside. In AD brain, a complex of GM1 and Aβ, termed “GAβ,” has been found to accumulate. In recent years, Aβ and GM1 have been identified in microdomains or lipid rafts. The functional roles of these microdomains in cellular processes are now beginning to unfold. Several articles also have documented the involvement of these microdomains in the pathogenesis of certain neurodegenerative diseases, such as AD. A pivotal neuroprotective role of gangliosides has been reported in in vivo and in vitro models of neuronal injury, Parkinsonism, and related diseases. Here we describe the possible involvement of gangliosides in the development of AD and the therapeutic potentials of gangliosides in this disorder.
Keywords: lipid raft, microdomain, amyloid β-protein
GANGLIOSIDES
Gangliosides are sialic acid-containing glycosphingolipids (GSLs) that are expressed in the outer leaflet of the plasma membrane of all vertebrate cells. Gangliosides are involved in a variety of functions, including serving as antigens, receptors for bacterial toxins, mediators of cell adhesion, and mediators and modulators of signal transduction (1). About 200 gangliosides are known today, differing in their carbohydrate components (2). Gangliosides are most abundant in the nervous system. Their expression in the nervous system is cell specific and developmentally regulated, and their quantities and species undergo dramatic changes during differentiation of the cell (3–5). Gangliosides also play an important role in the pathogenic mechanisms of many immune-mediated neurological disorders, such as Guillain-Barré syndrome (6). GM1 ganglioside is present in endocytic organelles, in the trans-Golgi network, and in the plasma membranes. More recently, it has been demonstrated that GM1 ganglioside is also present in nuclear membranes, adding another interesting facet to the myriad biological functions of gangliosides (7).
In the plasma membrane, where the bulk of GM1 is deposited, GM1 is not distributed uniformly but is more concentrated in noncoated invaginations (8). Gangliosides are also known to exist in clusters and to form microdomains or lipid rafts on the surface of the plasma membrane (9). This specific localization of gangliosides enables them to interact with a variety of bioeffectors, such as glycoproteins, antibodies, peptide hormones, and growth factors (10, 11). Furthermore, gangliosides can promote cell differentiation and induce neuritogenesis (12, 13). Gangliosides are also known to provide a neuroprotective role in in vivo and in vitro models of neuronal injury (14). The mechanisms of the neuroprotective effect of GM1 and related gangliosides, however, are still obscure (15).
ALZHEIMER'S DISEASE
Alzheimer's disease (AD), the most common form of dementia, is a progressive degenerative disease of the brain with loss of memory and cognition. AD is characterized neuropathologically by amyloid deposits or “senile plaques” (SPs), whose major component is an aggregated variant of amyloid β-protein (Aβ), a family of 39- to 42-residue peptides formed by normal sequential enzyme cleavage of the amyloid precursor protein (APP) (16, 17). In AD, there are also neurofibrillary tangles consisting of paired helical filaments containing hyperphosphorylated tau (18). Cleavage of APP by α-secretase produces a soluble form of APP (sAPPα), which precludes generation of Aβ (19). Cleavage of APP by the β-secretase, BACE1, at the N-terminus of the Aβ domain, however, generates sAPPβ and a C-terminal fragment, which undergoes a second cleavage by the protease γ-secretase to generate the soluble form of Aβ. Conversion of the soluble form of Aβ to the toxic variant with a high degree of β-sheet structures, a prerequisite for aggregation, is a critical step in the formation of amyloid deposits. It is not clear, however, what mechanism leads to the polymerization process in which normal soluble proteins are converted into insoluble aggregates or fibril forms. Because of this conversion, however, inhibition of Aβ aggregation and/or decomposition of fibrils formed in aqueous solution by small compounds, as well as inhibitors of β- and γ-secretases, have been studied extensively for the prevention and treatment of AD.
At present, the etiology of AD is not clearly understood, but it is likely to be multifactorial, including genetic predisposition, protein trafficking and turnover, GSL abnormalities, and impairment of neurotrophin signaling (20). In this review, we will focus on the GSL abnormalities that may be involved in the pathogenetic mechanisms of AD.
GANGLIOSIDE METABOLISM IN AD
The pathology of AD is closely related to lipid metabolism (21). During aging and neurodegeneration, the physicochemical properties of membranes are altered. This can result in imbalances in the proportion of lipids in membranes and/or changed ratios of membrane lipids, which may contribute to the pathogenesis of AD (22).
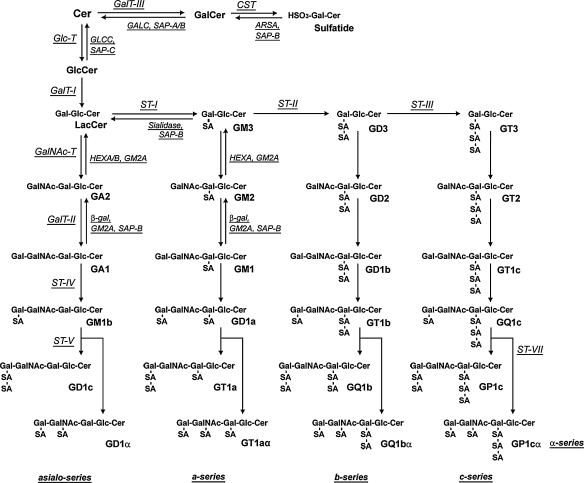
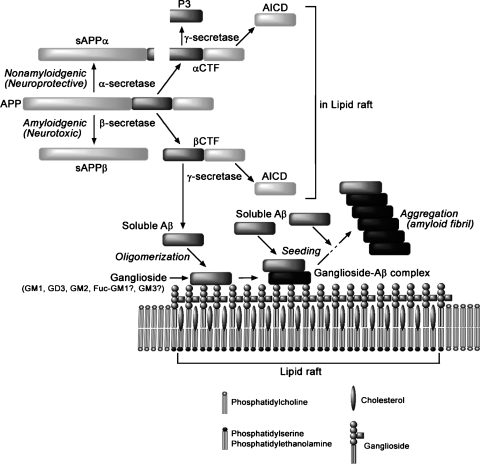
In brain cells, ganglioside and lipid abnormalities, in addition to pathogenic Aβ production, may contribute to the pathological conditions found in AD (20). Figure 1 shows the structure and metabolism of gangliosides in the brain. Several earlier studies showed alterations in ganglioside metabolism in AD brain (23–29). This is manifested as reductions in gangliosides in the majority of brain regions, including the cerebral cortex, hippocampus, basal telencephalon, and frontal white matter, and especially in the frontal cortex and white matter (24). The pattern of ganglioside alterations in AD differs according to age of onset. Specifically, the concentration of gangliosides is reduced to 58–70% of that of control brains in gray matter, and to 81% in frontal white matter, in early-onset or familial AD cases; however, gangliosides are significantly reduced only in the temporal cortex, hippocampus, and frontal white matter in late-onset cases (23). Brooksbank and McGovern (25) reported the ganglioside composition in the brains of adults with Down syndrome (DS) and in AD. DS is attributed to trisomy (triplication) of chromosome 21, the same chromosome on which APP is located. Although there were no abnormalities in the gangliosides of DS corpus callosum, the concentration of total gangliosides in frontal cortex and cerebellum of DS was reduced compared with controls. GT1b and GD1b showed the greatest loss, in contrast to a slight increase in GT1a, GD3, GM1, and GM2. Consistent with these changes, the ratio of total b-series to a-series gangliosides was reduced in DS brains. In contrast to the substantial changes in DS patients, the total gangliosides and their composition were normal in frontal cortex from patients with AD compared with age-matched controls, with the minor exception of reductions in the fractions of GQ1b and GT1L (25). Crino et al. (26) also reported that concentrations of gangliosides in individuals with dementia of the Alzheimer type (DAT) were significantly lower than the concentrations in control brains. Analysis of the ratios of a-series (GM1 and GD1a) to b-series (GD1b and GT1b) gangliosides revealed that DAT preferentially affected b-series gangliosides. The areas affected in DAT included the nucleus basalis of Meynert, and entorhinal, posterior cingulate, visual, and prefrontal cortices. Other studies, by Kracun and his coworkers (27–29), documented a significant decrease of ganglio-series gangliosides (GT1b, GD1b, GD1a, and GM1) in both the frontal and temporal cortex and basal telencephalon, as well as in the nucleus basalis of Meynert of the brains of patients with AD compared with those areas in control brains. Because neuronal loss and brain shrinkage are pathological hallmarks of AD and gangliosides are enriched in neurons, the reduced ganglioside concentrations of ganglio-series gangliosides are consistent with the pathological changes. Further study of the brains of patients with AD indicated that simpler gangliosides, such as GM2 and GM3, were elevated in both frontal and parietal cortices, which may coincide with accelerated lysosomal degradation of gangliosides and/or reactive astrogliosis occurring during neuronal death (29). These changes are also associated with AD.
Fig. 1.
Structure and metabolism of gangliosides in mammalian brain. The nomenclature of gangliosides follows the system of Svennerholm (183).
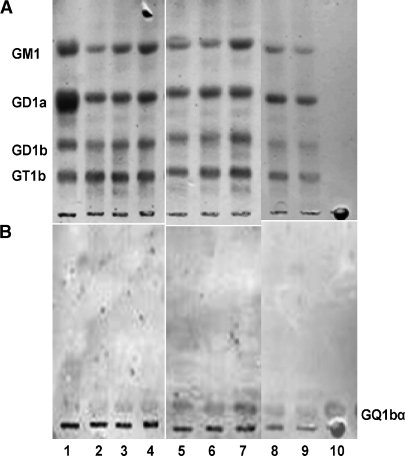
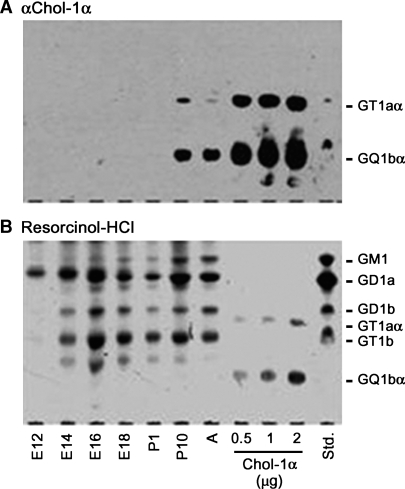
With respect to minor gangliosides, we have recently found GQ1bα expression in the brains of transgenic mice induced with AP-1 and Aβ1-42, and the content of this ganglioside increased during aging (Ariga et al., unpublished data; Fig. 2). GQ1bα is one of the so-called Chol-1 antigens that are markers of cholinergic neurons (30, 31). Expression of the Chol-1 antigens in rat brain regions, such as in the hippocampus, is developmentally regulated, and their concentrations increase with aging (32). In mouse brain, their expression in brain is present only during early prenatal stages characterized by rapid neuronal differentiation, presumably during development of the cholinergic neuronal system (5) (Fig. 3). There is a distinct possibility that upregulation of the expression of GQ1bα ganglioside in AD mouse brain could be a compensatory mechanism for the age-related decline of cholinergic function. These findings also suggest that abnormalities in gangliosides in the brains in individuals with AD or DS may reflect developmental disturbances.
Fig. 2.
Expression of GQ1bα in brains of transgenic (TG) mice induced with AP-1 and Aβ1-42. Lane 1: bovine brain ganglioside mixtures; lanes 2–4: TG (2 weeks); lanes 5–7: TG (4 weeks); lanes 8 and 9: wild-type; lane 10: GQ1bα standard, 10 ng. A: resorcinol-HCL reagent staining; B: immunostaining with anti-Chol-1 monoclonal antibody (CGR-41). The plates were developed with the solvent system of chloroform-methanol-0.2% CaCl2; 55:45:10; v/v/v.
Fig. 3.
Expression of GQ1bα in developing mouse brain. Gangliosides isolated from the developing mouse brains were subjected to high-performance thin-layer chromatography (HPTLC) immunostaining with GGR-41 anti-Chol-1α (GT1aα and GQ1bα) antibody (A), and then visualized with the resorcinol-HCl reagent (B) (5).
In the cerebrospinal fluid (CSF) of patients with AD, the total concentration of gangliosides did not differ significantly between the “probable AD” group and controls, but the distribution of ganglioside species underwent distinct changes. In the “probable AD” group, there was an increase in the amount of GM1 and GD1a, and a concomitant decrease in the amount of GD1b and GT1b. Although the pathogenetic mechanism for these changes in gangliosides in CSF in individuals with “probable AD” remains to be established, the changes may reflect the degeneration of nerve cells and synapses (33).
Immunohistological studies have been performed on the cellular and subcellular localizations of gangliosides in the brains of patients with AD using specific antibodies (34–36). In these cases of brain tissues from patients with AD-type dementia, some SPs were immunostained with anti-GD1a monoclonal antibody, with the strongest staining in the subiculum (34). However, no diffuse amyloid deposits, neurofibrillary tangles, or neuropil threads were immunostained. Antibodies to c-series gangliosides and microtubule-associated protein 5, both of which are preferentially expressed in fetal brains, immunostained the dystrophic neurites associated with SPs, neurofibrillary tangles, and neuropil threads. All of these pathological hallmarks of AD are abundant in cortical layers 3 and 5 (35). In AD, many neurofibrillary tangles, neuropil threads, and dystrophic neurites react strongly with A2B5 (36). Although the amount of gangliosides and their patterns are similar in control and in AD cases (37), an observation was made that the A2B5 antibody reacted with human brain c-series gangliosides and, unexpectedly, sulfatides (38).
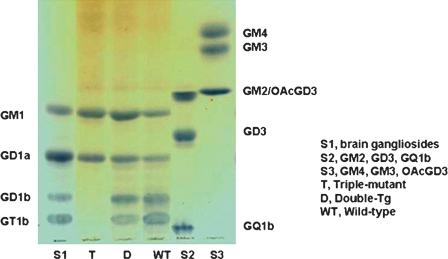
The brain gangliosides of different transgenic mouse models of AD have been analyzed and compared with those of age-matched wild-type mice (39–42). Barrier et al. (39) observed a marked increase in the simple gangliosides GM2 and GM3 in the cortex of 2-year-old APPSL mice expressing the Swedish (K670N/M671L) and London (V717I) mutations of human APP. The proportions of GQ1b, GD1b, GD3, and GT1a were all moderately but significantly decreased in these mice, compared with wild-type controls. Cerebellar ganglioside levels were unchanged. Cortical GM2 and GM3 were also significantly increased, as was GD3, in APPSL transgenics co-expressing either a point mutation (M146L) in presenilin 1 (PSEN1) or a homozygous knock-in of the M233T/L235P PSEN1 mutation. Additionally, a loss of complex gangliosides (GT1a, GD1a, and GM1) was noted in the brains of APP/PSEN1 knock-in mice, but not in the APP/PSEN1 double-transgenic mice. Interestingly, although cortical levels of GM2 and GM3 are expressed at very low levels in wild-type mouse cortex, they were reduced even further in both PSEN1 mutant lines in the absence of the APPSL transgene. Thus, ganglioside alterations occurring in mice expressing the human mutant APPSL transgene with or without a PSEN1 mutation, but not with a PSEN1 mutation alone, are similar to those observed in humans with AD (23, 28, 39). In contrast to the robust changes in the cortex, only slight changes in the ganglioside pattern were found in the cerebellum in the PSEN1 and APP/PSEN1 mutant lines (40). Alterations in cerebellar gangliosides have not been reported in the cerebellum of AD brains, although b-series cerebellar gangliosides are reduced in normal aging (29) and a reduction in total cerebellar gangliosides has been reported in patients with DS (25). Recently, Bernardo et al. (40) reported ganglioside alterations in mixed cortical/hippocampal tissue of mice lacking St8sia1, the gene that codes for GD3 synthase (GD3S), and in a double-transgenic (APPSwe/PSEN1Δe9) mouse model of AD cross-bred with GD3S−/− mice. GD3S is the biosynthetic enzyme that converts GM3 to GD3 and is required for synthesis of b-and c-series gangliosides (STII in Fig. 1). Brain ganglioside levels were measured in double-transgenic, triple-mutant, and wild-type mice. The triple-mutant (APP/PSEN1/GD3S−/−) mice had a complete lack of b-series gangliosides and a corresponding increase in GM1 (63.8%) and GD1a (50.8%), compared with the levels in wild-type mice (Fig. 4). No significant differences in four major brain gangliosides were found between wild-type and APP/PS-1 double-transgenic mice. Neither tissue cholesterol nor fatty acid phospholipids differed significantly across the four groups. These APP/PSEN1 double-transgenics exhibit robust impairments on a number of reference memory tasks (40). In contrast, APP/PSEN1/GD3S−/− triple-mutant mice performed as well as wild-type control and GD3S−/− mice. Consistent with the cognitive improvements, Aβ plaques and associated neuropathology were almost completely eliminated in the APP/PSEN1/GD3S−/− mice. These results suggest that GD3 synthase may be a novel therapeutic target to combat the cognitive deficits, amyloid plaque formation, and neurodegeneration that afflict Alzheimer's patients. It is of note that the APP/PSEN1 bigenic mice have significantly impaired memory despite having no significant changes in major brain gangliosides, but the GD3S−/− mice have normal memory in the presence of substantial redistribution of gangliosides. This suggests that the ganglioside alterations per se are not directly responsible for the cognitive effects in this study, with the possible exception of the putative neutrophic effects of elevated GM1. Instead, the memory deficits in APP-overexpressing mice have been attributed to small Aβ oligomers. Thus the cognitive effects of the ganglioside alterations are probably attributable to reduced Aβ oligomerization in these mice, secondary to lower levels of APP protein and reduced Aβ-binding substrate (b-series gangliosides). These transgenic models represent valuable tools for further investigation of the role of altered ganglioside metabolism in the pathogenesis and treatment of AD (39, 40).
Fig. 4.
Representative thin-layer chromatographs from wild-type, double-transgenic, and triple-mutant mice. The plate was developed with the solvent system of chloroform-methanol-0.2%CaCl2; 55:45:10; v/v/v). Triple-mutant mice lack two of the four major brain gangliosides, and have increased levels of a-series gangliosides GM1 and GD1a, compared with wild-type mice. Differences between double-transgenic and wild-type mice were not significant.
With respect to the involvement of catabolic enzymes for gangliosides, Pitto et al. (43) reported enhanced GM1 ganglioside catabolism in cultured fibroblasts from patients with AD. After cultured fibroblasts were incubated with GM1 ganglioside [3H]radiolabeled at the sphingosine moiety, they found that the content of tritiated GM2 and GM3 gangliosides was increased in AD fibroblasts compared with that in control cells. The increase in the concentrations of GM2 and GM3, in comparison with control cells, could be due to the increased activities of acidic β-d-galactosidase, which is responsible for hydrolyzing GM1 to GM2 in AD fibroblasts. Kalanj-Bognar et al. (44) also reported a statistically significant increase of β-galactosidase activity in leukocytes of both DAT and DS. In addition, sialyltransferase activity was reduced in the frontal and temporal cortical lobes and serum of Alzheimer's patients. This may be a specific biochemical event associated with the AD-like neurodegeneration, although no change was observed in the hippocampus (45, 46). This significant decrease in the serum sialyltransferase activity may prove to be a useful early biochemical marker of neurodegeneration and may provide an indication of the underlying cellular events that occur during the process of nerve cell death in AD (46). Cataldo et al. (47) found that lysosomal hydrolases, cathepsin D, and β-hexosaminidase A were colocalized with Aβ in a subgroup of diffuse plaques in the cerebellum and striatum of individuals with AD or DS. It is suggested that the activities of several lysosomal enzymes involved in catabolism of gangliosides may be taken as a peripheral hallmark of AD or DS patients.
Gangliosides are known to play an important role in neuronal development and regeneration, whereas anti-ganglioside antibodies have been shown to impair these processes. For example, passive transfer of anti-GD1a antibody severely inhibited axon regeneration after peripheral nervous system injury in mice (48). Anti-ganglioside antibodies have been described in sera of patients with peripheral neuropathy and a number of immune-mediated neurological diseases (6, 49). Chapman et al. (50) examined neuronal degeneration in AD and noted that it was associated with the presence of anti-ganglioside antibodies. A significantly elevated level of antibodies specific to ganglioside GM1, but not to other gangliosides (GD1a, GD1b, GT1b, and GQ1b), was found in patients with AD compared with normal age-matched controls. In addition, a high level of antibodies to GM1 was also found in patients with multi-infarct dementia and Parkinson's disease with dementia but not in nondemented patients with other neurodegenerative diseases. AD is associated with degenerative changes in the nuclei of basal forebrain that provide most of the cholinergic input to the cortex and hippocampus and with a reduction in presynaptic cholinergic parameters in these areas. Interestingly, Chapman et al. (51) reported the presence of antibodies in sera of AD patients that bind specifically to cholinergic neurons. Our preliminary results indicated that a cholinergic neuron-specific ganglioside (GQ1bα) is elevated in an AD model of transgenic mice (Ariga et al., unpublished results), which is consistent with this view. These observations may reflect a specific change in ganglioside metabolism that is associated with neurodegenerative processes underlying AD and other forms of dementia.
Interaction of Aβs with gangliosides
A fundamental question concerning the pathogenic mechanism of AD is how nontoxic Aβ is converted to its toxic aggregates in the brain (52). Interactions between Aβs and neuronal membranes have been postulated to play a pivotal role in the neuropathology of AD. Since Terzi, Holzemann, and Seelig (16) first reported that Aβ undergoes a conformational transition from random coil to ordered structure rich in β-sheet after addition of lipid vesicles containing negatively-charged lipids, several studies have demonstrated that Aβs bind to gangliosides, especially GM1, resulting in an altered secondary structure (53, 54). Aβ-ganglioside interaction may be implicated in formation of Aβ polymerization and/or fibrinolysis (54). Further studies have indicated that conformational changes in Aβ (16, 54–56) depend on several factors, including pH, the presence of metal ions (57, 58), and the concentration of gangliosides (54). Aβ binds to membranes containing ganglioside GM1, and upon binding, undergoes a conformational transition from random coil to an ordered structure rich in β-sheet. This interaction appears to be ganglioside specific because no changes in Aβ1-40 conformation were found in the presence of various phospholipids or sphingomyelin. The peptide binds selectively to membranes containing gangliosides with a binding affinity ranging from 10−6 to 10−7 M, depending on the type of the ganglioside sugar moiety. This interaction appears to be ganglioside specific under experimental conditions involving neutral pH and physiologically relevant ionic strength (54). On the other hand, the isolated oligosaccharide moiety of the ganglioside is ineffective in inducing alterations in the secondary structure of Aβ1-40 (54, 59), suggesting the involvement of the lipid component in the interaction. In addition, McLaurin and Chakrabartty (55) reported that Aβ1-40/Aβ1-42 disrupts acidic lipid membranes, and this disruption is greater at pH 6.0 than at pH 7.0. At pH 7.0, gangliosides induce Aβ1-40/Aβ1-42 to adopt a novel α/β conformation. They speculated that gangliosides can sequester Aβ and thereby prevent β-structured fibril formation; alternatively, gangliosides may be involved in normal Aβ functioning and/or clearance. This effect appears to be ganglioside specific, because under the conditions of physiologically relevant ionic strength, no changes in Aβ conformation could be detected in the presence of ganglioside-free vesicles composed of zwitterionic or acidic phospholipids. Further study indicated that binding of Aβ1-40 to mixed gangliosides or GM1-containing vesicles induced an α-helical structure at pH 7.0 and a β-structure at pH 6.0 (56). Notably, the interaction of Aβ with ganglioside clusters is stronger under physiological conditions, and therefore is physiologically important (60). During incubation with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine vesicles containing physiological levels of GM1, the association of Aβ with vesicles seeds the formation of Aβ fibrils. Thus, favorable interactions between Aβ and GM1 in the cell membrane provide a mechanism for Aβ fibrillogenesis in vivo, and Aβ-induced disruption of the cell membrane may provide a pathway by which Aβ exerts toxicity (61).
Emerging evidence has indicated that disruption of the homeostatic balance of redox-active biometals such as Cu and Fe can lead to oxidative stress, which plays a key role in the neuropathology of AD. Atwood et al. (57) reported that unlike other biometals tested at maximal biological concentrations, marked Cu2+-induced aggregation of Aβ1-40 occurred as the solution pH was lowered from 7.4 to 6.8 and that the reaction was completely reversible with either chelation or alkalinization. The aggregation-inducing activity of metals is in the following order: Cu2+ > Fe3+ > or = Al3+ > Zn2+ (58). Interestingly, incorporation of ganglioside GM1 into the membrane of liposomes enhances liposomal aggregation induced by Aβ as well as other peptides (62).
Using surface plasmon resonance (SPR), Valdes-Gonzalez, Inagawa, and Ido (63) investigated the interaction of Aβ1-42 and seven different neuropeptides with membranes containing gangliosides. A wide range of affinities with dissociation constants of KD = 10−3–10−7 M characterize the bindings with the following scheme: for GM1, Aβ1-42 > DYN > SP = GAL = SOM = BRD > OXY = ENK; for GD1a, Aβ1-42 = DYN = GAL > SP = SOM = BRD = OXY > ENK; and for GT1b, Aβ1-42 > DYN > SP = GAL > SOM = BRD = OXY > ENK. The binding specificities of Aβs such as Aβ1-40, Aβ1-42, Aβ40-1, Aβ1-38, Aβ25-35, and β-APP analogs for different GSLs have also been determined by SPR using a liposome capture method (11). Aβ1-42, Aβ1-40, Aβ40-1, and Aβ1-38, but not Aβ25-35, bind to GM1 ganglioside with affinities in the following order, from greatest to lowest: Aβ1-42 > Aβ40-1 > Aβ1-40 > Aβ1-38. Aβ-APP analogs bind to GM1 ganglioside with a relatively lower affinity. Aged derivatives of Aβs were found to have higher affinity to GM1 ganglioside than freshly prepared or soluble derivatives. Aβ1-40 binds to a number of gangliosides with the following order of binding strength: GQ1bα > GT1aα > GQ1b > GT1b > GD3 > GD1a = GD1b > LM1 > GM1 > GM2 = GM3 > GM4. Neutral GSLs have a much lower affinity for Aβ1-40 than gangliosides. The results suggest that an α2,3NeuAc residue on the neutral oligosaccharide core of gangliosides is required for binding. In addition, the α2,6NeuAc residue linked to GalNAc in the α-series of gangliosides contributes significantly to the binding affinity for Aβ. Specific binding between Aβ1-40 and ganglioside GM1 in the lipid raft-like domain of the membrane is suggested from an SPR experiment. The difference in binding ability to the cell membranes accounts for the cytotoxicity of Aβ1-40; namely, aggregated β-sheet Aβ1-40 presents higher cytotoxicity than random coil Aβ1-40 (64).
With respect to the mode of interaction between Aβ and gangliosides, the ganglioside sugar moiety, specifically the sialic acid, plays an important role. NMR spectroscopic studies of ganglioside-Aβ interactions conducted in sodium dodecyl sulfate micelles, a membrane-mimicking environment, revealed that asialo-GM1 bound with Aβ in a manner that could prevent β-sheet formation; ganglioside GT1b, however did not show such a property (65). Williamson et al. (66) also demonstrated with NMR studies that interaction of 15N-labeled Aβ1-40 and Aβ1-42 with GM1 micelles is localized to the N-terminal region of the peptide, particularly residues His13 to Leu17, and the peptide becomes more helical upon binding. The key interaction is with His13, which causes the peptide to undergo a GM1-specific conformational change. The sialic acid residue of the ganglioside headgroup is important for determining the nature of the conformational change. The isolated pentasaccharide headgroup of GM1 alone, however, is not bound, suggesting the need for a polyanionic membrane-like surface. Several studies have demonstrated that residues 12–28 of Aβ are the most likely targets for stimulating fibrillogenesis of this peptide when stimulated 10-fold by apolipoprotein E (apoE). This portion of Aβ has also been shown to be important for the adoption of a β-pleated sheet structure (67). In addition, Aβ residues 1–35 are required for the binding of the Aβ peptide to authentic amyloid plaques, as shown in an NMR study (68). More recent studies indicated that the binding site for GM1 was located within residues 52–81 (N-terminus) of APP, resulting in a conformational change of APP (69). This phenomenon is specific for GM1, but not for GD1a, GT1b, and ceramide, indicating that specific binding depends on the sugar moiety of GM1.
Interaction of Aβ with gangliosides may play a critical role in the pathogenesis of AD
In brains of patients with AD, the major component of amyloid deposits is known to be a 39- to 43-residue peptide (16). In CSF, Aβ1-40 is the major soluble species (70). Aβ1-42, on the other hand, is a minor soluble species that is more fibrillogenic than Aβ1-40 (71). Unexpectedly, monomeric Aβ1-40 serves as an antioxidant molecule and plays a neuroprotective role in Aβ1-42-induced neurotoxity in cultured rat brain cells (71).
The conversion of soluble, nontoxic Aβ to aggregated, toxic Aβ rich in β-sheet structure by seeded polymerization has been presumed to be a key step in the development of AD (72, 73). Choo-Smith et al. (54) reported that addition of ganglioside-containing vesicles to the peptide solution dramatically accelerates the rate of fibril formation compared with vesicles without gangliosides. The mechanism of ganglioside-mediated Aβ fibrillization probably involves an initial step in which the GSL-bound peptide self-associates on the membrane surface, undergoing a conformational transition to a β-sheet structure. Surface-associated, β-sheet-rich, peptide micro-aggregates could then act as a specific template (“seed”) to recruit additional peptide molecules from solution and promote fibril formation by a β-sheet augmentation mechanism (54). Aβ aggregation in brain is accelerated through an increase in the level of GM1 in neuronal membranes (42). Further studies have demonstrated that GM1 and GT1b promote the aggregation and cytotoxicity of Aβ1-40, and these gangliosides, especially GM1, catalyze the formation of neurotoxic fibrils (74). The effect is dose dependent; in the presence of lower concentrations of GM1 (about 25 μM), Aβ1-40 aggregates much more slowly, indicating that an increase in the concentration of GM1 significantly facilitates the aggregation of Aβ. An increase in the cholesterol concentration in the neuronal membranes also accelerates Aβ aggregation through the formation of endogenous seeding (75, 76), suggesting that cholesterol is a risk factor for AD development. These results further underlie the importance of control of cellular cholesterol and/or ganglioside contents in the pathogenesis of AD.
Accumulation of specific ganglioside-bound Aβ complex in AD brain
As indicated above, gangliosides can mediate Aβ1-40 secretion and accumulation in the brain, which may be involved in the pathogenesis of AD. Yanagisawa et al. (77) found that GM1 ganglioside bound to Aβ1-42, but not to Aβ1-40, to form a complex termed “GAβ” in AD brains. GAβ has unique characteristics, including an extremely high aggregation potential and an altered pattern of immunoreactivity, which results in seeding for amyloid fibril formation in brains. The formation of GAβ serves as one of the critical factors in the development of AD and may provide new insights into its pathophysiology (78). Recently, Kimura and Yanagisawa (79) reported, based on an immunohistochemical analysis, the accumulation of GAβ immunoreactivity in sections of cerebral cortices of cynomolgus monkeys of different ages, from 4 to 36 years old. The accumulation occurs exclusively in subcellular organelles involved in the endocytic pathway, including early, late, and recycling endosomes, but not in those involved in the secretory pathway. Because Aβ generation and GM1 accumulation probably occur in early endosomes, the results provide further evidence that endosomes are intimately involved in the Aβ-associated pathology of AD (79). The occurrence of GAβ in AD brain was further confirmed biochemically by staining with cholera toxin-B subunit (Ctxb), which preferentially binds to GM1, and by immunoprecipitation experiments using several anti-Aβ monoclonal antibodies (80). The assembly of wild-type and Arctic-, Dutch-, and Flemish-type mutant Aβs is accelerated in the presence of not only GM1, but also GM3 and GD3 gangliosides. Notably, all of these Aβs accelerate the assembly of different types of Aβ aggregates, following prior binding to a specific ganglioside (81). For instance, Dutch- and Italian-type Aβs require GM3 ganglioside for their assembly (82). The Arctic-type Aβ, in contrast to the wild type and other variant forms, shows a markedly rapid and higher level of amyloid fibril formation in the presence of sodium dodecyl sulfate or GM1 ganglioside (83). Recent studies indicate that a toxic, soluble Aβ assembly is formed in the presence of liposomes containing GM1 ganglioside more rapidly and to a greater extent from a hereditary variant-type (“Arctic”) Aβ than from wild-type Aβ (84). Toxic soluble Aβ is also formed from soluble Aβ through incubation with natural neuronal membranes prepared from aged mouse brains in a GM1 ganglioside-dependent manner. These examples suggest that local gangliosides play a crucial role in the region-specific Aβ deposition in the brain.
The role of GSLs in APP processing in AD brain
The exact role of GSLs, particularly gangliosides, on APP processing has not been fully clarified. Zha et al. (85) reported that exogenously added GM1 promoted Aβ biosynthesis and decreased soluble APPα secretion in SH-SY5Y and COS7 cells stably transfected with human APP695 cDNA, suggesting a regulatory role for GM1 in the APP processing pathway. Importantly, GM1 increased α-secretase cleavage products as well, indicating that the effect on APP processing was not a specific increase in BACE1 activity. Tamboli et al. (86) demonstrated that GSLs were implicated in the regulation of proteolytic processing and subcellular transport of APP. Inhibition of GSL biosynthesis reduced the secretion of sAPP and Aβ in different cell types, including human neuroblastoma SH-SY5Y cells, whereas the addition of exogenous brain gangliosides reversed these effects. Biochemical and cell biological experiments demonstrated that pharmacological reduction of cellular GSL levels inhibits maturation and cell surface transport of APPβ. In the GSL-deficient cell line GM95, cellular levels and maturation of APPβ are also significantly reduced as compared with normal B16 cells. Thus, enzymes involved in GSL metabolism might represent targets to inhibit the production of Aβs. In the present context, Sawamura et al. (87) found that in GSL-deficient cells, the secretion of sAPPα and the generation of a C-terminal fragment cleaved at the α-site dramatically increased, whereas β-cleavage activity remained unchanged and the epsilon-cleavage activity decreased without alteration of the total APP level. Thus, the cellular levels of GSLs may be involved in the pathological process of AD by modulating APP cleavage. Kitazume et al. (88) reported that BACE1 is involved in the proteolytic cleavage and secretion of a Golgi-resident sialyltransferase, β-galactoside α2,6-sialytransferase (ST6Gal I) that produces a sialyl α2,6-galactose residue, suggesting the possible existence of other glycosyltransferases in the rafts. ST6Gal I secretion is increased in BACE1-transgenic mice and decreased in BACE1-knock-out mice (89), confirming the involvement of BACE1 in ST6Gal I secretion in vivo. These data indicate that enhancement of BACE1 activity may down-regulate APP sialylation to attenuate Aβ production, thereby contributing to prolonging the course of disease development (90).
Interaction of gangliosides and Aβs in neuronal cells
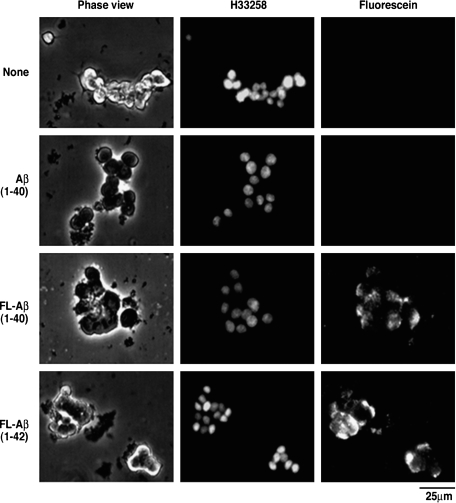
PC12 pheochromocytoma cells (PC12 cells) derived from an adrenal medulla pheochromocytoma in the New England Deaconess Hospital strain of rats (91) have been widely used as a model system for studying neuronal functions, because they develop neurite-like processes when exposed to nerve growth factor (NGF) (92). Interestingly, GM1 has been shown to increase the viability of PC12 cells exposed to hydrogen peroxide or Aβ25-35 (15). PC12 cells transfected with a mutant gene expressing the Swedish APP double mutation were more sensitive to oxidative stress than cells transfected with wild-type APP or vector-transfected cells. GM1 enhanced the viability of the cells transfected with the mutant gene. Exposure to hydrogen peroxide or Aβ25-35 results in a partial inactivation of Na+, K+-ATPase and increases malondialdehyde accumulation in PC12 cells. Several papers have documented the involvement of GM1 ganglioside or cholesterol in amyloid fibril formation in PC12 cells. Wang, Rymer, and Good (93) reported that reduction of either cholesterol or membrane-associated sialic acid residues in PC12 cells significantly reduced Aβ-induced GTPase activity, a mechanism implicated in Aβ toxicity. Wakabayashi and Matsuzaki (94) reported the effect of Aβ1-42 in PC12 cells with or without NGF-induced differentiation. Aβ1-42 was weakly cytotoxic to untreated cells, the viability of which was 70% even after 72 h incubation. In contrast, Aβ1-42 was remarkably cytotoxic to NGF-differentiated PC12 cells. After 24 h incubation, the viability was reduced to 56%, and at 72 h of incubation, the viability was further lowered to 28%. Further studies indicate that Aβ aggregation also occurs in lipid rafts mediated by a cluster of GM1, resulting in Aβ-induced toxicity in PC12 cells (95). Yuyama, Yamamoto, and Yanagisawa (96) reported that chloroquine treatment induced accumulation of GM1 in PC12 cells, resulting in accelerated amyloid fibril formation from soluble Aβ. Wakabayashi et al. (97) reported the interaction of fluorescein-labeled Aβ (FL-Aβ) with PC12 cells using confocal laser microscopy. FL-Aβ colocalized with GM1-rich domains on cell membranes and accumulated in a concentration- and time-dependent manner, leading to cytotoxicity. Yamamoto et al. (98) investigated whether GM1 ganglioside on the surface of PC12 cells is sufficiently potent to induce the assembly of an exogenous soluble Aβ. A marked Aβ assembly was observed in the culture of NGF-treated PC12 cells. Notably, immunocytochemistry revealed that despite the ubiquitous surface expression of GM1 throughout cell bodies and neurites, Aβ assembly initialy occurred at the terminals of SNAP25-immunopositive neurites. Aβ assembly in the culture was completely suppressed by coincubation of Aβ with Ctxb, a natural ligand for GM1 ganglioside, or 4396C, a monoclonal antibody specific to GAβ.
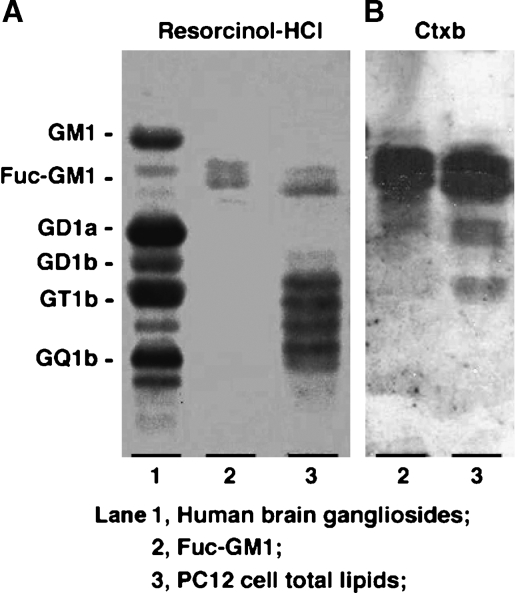
A caveat to the above studies on detection of GM1 using Ctxb (96–98) is that Ctxb is not specific to GM1. In fact, it is widely known that Ctxb also interacts, albeit with a lower affinity, with a wide variety of ligands, including fucosyl-GM1, GD1b, and even lipid A (99, 100). Thus, despite the claim that Aβ interacts with GM1 in PC12 cells to provide “seeding” for amyloid to accumulate, we contend that Aβ is most likely interacting with fucosyl-GM1 in this cell line (Fig. 5), based on a series of immunocytochemical and biochemical studies (101). This is because differentiated PC12 cells express fucosyl-GM1 with no or very little GM1, and it is the former that interacts with this ligand (see Fig. 6), as reported previously (102–105). Thus, interaction of Aβ with gangliosides to effect amyloid accumulation may not be limited to GM1; indeed, other gangliosides are also involved, as shown by Yanagisawa, Ariga, and Yu (101).
Fig. 5.
Accumulation of Aβ in PC12 cells expressing no GM1. PC12 cells were cultured in the presence or absence of Aβ1-40 (2.5 μg) or FL-Aβ (1-40 or 1-42) (2.5 μg) for 48 h. The nuclei were stained with Hoechs 33258 (2 μg/ml) (101).
Fig. 6.
Reactivity of cholera toxin B-subunit (Ctxb) in PC12 cells. PC12 cells did not express GM1 (A), but expressed FucGM1 that reacted with Ctxb (B). Total lipids extracted from the PC12 cells were subjected to HPTLC-overlay assay with biotin-conjugated Ctxb (2.5 μg/ml). Resorcinol-HCl reagent was used for ganglioside detection. Lane 1: human brain ganglioside mixture; lane 2: authentic sample of fucosyl-GM1 (FucGM1); lane 3: total lipids extracted from PC12 cells (101).
Concerning the mechanism of action of Aβ peptides, it has been shown that treatment of the human monocytic cell line THP-1 with Aβ1-40 or Aβ25-35 resulted in increased release of cytotoxic cytokines such as interleukin (IL)-1β, IL-6, and TNF-α from these cells (106). However, treatment of Aβ-activated THP-1 cells with GM1 or several other gangliosides, but not neutral GSLs, significantly decreased the release of these cytokines (106). Furthermore, GM1 stimulates the expression of ubiquilin 1 in human neuroblastoma cells and rat cortical neurons, whereas other gangliosides and asialo-GM1 do not. Ubiquilin 1 is a candidate gene for AD and has been shown to modulate components of the γ-secretase complex in the proteolytic processing of APP. Thus, further investigation of the interaction of GM1 and ubiquilin 1 in the etiology and pathology of AD is warranted (107). Whether ubiquilin acts to modulate cytotoxic cytokines is another possibility that remains to be elucidated.
MICRODOMAINS OR LIPID RAFTS
During the early 1990s, the concept of membrane “flask-shaped” morphological units, termed “caveolae,” was introduced. These membrane microdomain structures are characterized by the presence of the scaffold protein caveolin (8, 108), and they are enriched with various lipids, such as cholesterol, GSL, and sphingomyelin. The concentrations of GM1 ganglioside and cholesterol are higher in the membrane domains than in any other membrane substructures (8). There are numerous examples of such a raft clustering process, one of which is the formation of caveolae. Caveolae are surface invaginations, 50 to 100 nm in diameter, containing raft lipids and the protein caveolin. The caveolins form oligomers, and formation of caveolae is probably driven by protein polymerization in the cellular membranes (109). These specialized membrane domains are also known as “lipid rafts” (110). The original concept of rafts was used as an explanation for the transport of cholesterol from the trans-Golgi to the plasma membrane. Increasing evidence implicates lipid rafts not only in the sorting and trafficking of biomaterials through the secretary and endocytic pathways, but also in lipid homeostasis and tumorigenesis. Together with many signaling molecules, lipid rafts are implicated as platforms for assembly and launching of signaling cascades (9). Thus, lipid rafts contain not only lipid components, but also numerous signaling molecules such as glycosylphosphatidylinositol (GPI)-anchored proteins and receptor- or nonreceptor-type tyrosine kinases, which modulate various physiological processes in cells (9).
Lipid rafts are too small to be seen by standard microscopic techniques. It is impossible to isolate lipid rafts in their native state. Detergent-resistant membranes (DRMs), containing clusters of many rafts, can be isolated by extraction with Triton X-100 or other detergents (9). Because of their detergent resistance and composition, lipid rafts are also referred to as glycolipid-enriched membranes (GEMs) (111), glycosynapses (108), detergent-insoluble glycolipid-enriched membranes (112), or DRMs. If cholesterol is extracted by methyl-β-cyclodextrin or complexed by saponin, the raft proteins usually, but not always, become detergent soluble (113). The concept of microdomains mediating cell adhesion, particularly carbohydrate-dependent adhesion coupled with signaling, was developed only recently, and these domains are termed “glycosynapses” (108, 114). Hakomori (108) defined the microdomains as caveolae/rafts containing cholesterol/sphingomyelin-enriched membranes and caveolin. A glycosynapse is enriched in clustered glycoconjugates in a GEM to which glycoconjugate ligands bind. The glycoconjugates may be GSLs, O-linked glycoproteins, or N-linked receptor-type glycoproteins, which are associated with signal transducers, tetraspanins (including proteolipid proteins), and membrane lipids.
Various methods used to isolate or distinguish membrane microdomains have been devised during the past decade, although none of them are satisfactory for defining microdomains with subtle differences in chemical composition and physical properties (115). Triton X-100 has been the most popular detergent in the isolation of microdomains. Many investigators have used the zwitterionic detergent CHAPS as an alternative to Triton X-100 to generate DRMs (116). Recently, the detergents polyoxyethylene 10 oleyl ether (Brij96) and polyoxyethylene 20 oleyl ether (Brij98) have been used for isolation of lipid rafts (117). The DRM fractions isolated using Triton X-100 are considerably heavier than those isolated from homogenates treated with Brij96 (118).
Membrane lipid rafts are reported to be heterogeneous in size and composition (117, 119–121), which is probably the result of using different isolation procedures. Lipid rafts of different lipid and protein compositions appear to be localized to different regions of cells (121) and vary in size distribution (119). Rouvinski et al. (116) pointed out the size heterogeneities of three CHAPS-insoluble complexes that all contain cholesterol and GM1. Large complexes are enriched in caveolin, and medium-size complexes are enriched in prion protein. APP is primarily confined to small assemblies. The distribution of GD3 and GM1 gangliosides within rafts, either isolated from rat cerebellum or in intact cerebellar granule neurons, has been found to be different, suggesting yet another type of heterogeneity for rafts (120). In addition, rafts have been found to consist of multilayer components; uropod rafts (U rafts) at each cell pole are enriched in GM1, whereas leading-edge rafts (l rafts) are enriched in GM3 (122).
The functional roles of microdomains or lipid rafts in cellular processes are now beginning to unfold. In recent years, several articles have documented the involvement of these microdomains in the pathogenesis of certain neurodegenerative diseases, such as AD, prion disease, HIV-1, and acquired-immunodeficiency syndrome (113, 123).
AD-related components in membrane microdomains or lipid rafts
There is increasing evidence that lipid rafts are involved in a multitude of cellular functions and may be targets of neurodegenerative diseases such as prion disease and AD (113). In AD, lipid rafts may play an important role in proteolytic processing and regulation of APP cleavage. Several reports have documented that lipid rafts or DRMs from cultured cells and mammalian brains contain many AD-related lipids (see Table 1). GM1 and other gangliosides are present in highly organized and functionally essential microdomains (lipid rafts) of neuronal membranes, together with cholesterol and phospholipids (43, 112, 120, 124, 125). Fucosylated gangliosides, such as α-fucosyl and α-galactosyl-GM1, have been found in lipid rafts of PC12 cells (105). Ceramide (126) and ceramidase (127) are reported as components of lipid rafts. Neutral ceramidase was found to be enriched in the raft microdomains with cholesterol and GM1 (127). Most of the neutral sphingomyelinase activity is in the rafts, suggesting that the conversion of sphingomyelin to ceramide in rafts is an important event in neural cell apoptosis (126). Wang et al. (128) reported a close association of the Neu3 (the ganglioside-specific) sialidase with caveolin-rich microdomains using HeLa cells and Neu3-transfected COS-1 cells. Plasma membrane ganglioside sialidase has been proven as a marker of lipid rafts and is codistributed with the raft markers ganglioside GM1, flotillin, Src family kinases, and GPI-anchored protein (125).
TABLE 1.
Raft components related to Alzheimer's disease
| Components | Tissues/cells | References |
|---|---|---|
| GM1 | Synaptosomes in apoE-knock-in | 42 |
| Mouse brain | ||
| Plasma membrane | 125 | |
| Rat kidney | 127 | |
| GM1, GM3, and globoside | Neuro2a cells | 111 |
| GM1 and GD3 | Rat cerebeller granule neurons and cerebellum | 120 |
| GM1 and GM2 | AD brain | 124 |
| Fucogangliosides | PC12 cells | 105 |
| Ceramide | LA-N-5, F11, and HOG cells | 126 |
| Ceramidase | Rat kidney | 127 |
| Ceramidase and sphingomyelinase | LA-N-5, F11, and HOG cells | 126 |
| Sialidase | Plasma membrane | 125 |
| Sialidase (Neu3) | HeLa cells, COS-1 cells | 128 |
| Amyloid β-precursor protein (APP) | Rat brain | 129 |
| Cerebral cortex of AD brain | 130 | |
| Madin-Darby canine kidney and P19 cells | 131 | |
| Neuro2a cells | 132 | |
| BASE1-HEX cells | 133 | |
| APP-transfected cells | 134 | |
| AD brain | 135 | |
| SH-SY5Y neuroblastma cells | 136 | |
| C2C12 cells | 137 | |
| APP C-terminal fragments (CTF2) | Mouse brain | 138 |
| APP-transfected cells | 134 | |
| APP N-terminal fragments (sAPP) | Epithelial cells | 139 |
| Amyloid β-protein (Aβ) | Rat brain | 129 |
| SH-SY6Y neuroblastma cells | 140 | |
| Tg2576-transgenic mouse (AD) | 141 | |
| Aβ dimers | Tg2576-transgenic mouse (AD) | 141 |
| Aβ oligomers | C2C12 cells | 137 |
| β-secretase (BACE1; Asp2) | Tg2576-transgenic mouse (AD) | 141 |
| SH-SY5Y neuroblastma cells | 145 | |
| SH-SY5Y neuroblastma cells | 146 | |
| Mouse embryonic fibroblasts | 21 | |
| BASE1-HEX cells | 133 | |
| A high-molecular-weight complex of BACE | AD brain | 135 |
| α-Secretase | COS7 transfectants | 142 |
| CHO transfectants | 143 | |
| Neuro2a cells transfected with PSEN1 | 144 | |
| Mouse brain | 138 | |
| γ-Secretase (PSEN1, presenilin 1) | Rat brain | 129 |
| Cerebral cortex of AD brain | 147 | |
| APP-transfected cells | 134 | |
| BASE1-HEX cells | 133 | |
| Mouse brain | 138 | |
| ApoE | Tg2576-transgenic mouse (AD) | 141 |
| Tau | Tg2576-transgenic mouse (AD) | 141 |
| Plasmin | Hippocampal neurons of AD brain | 148 |
| Rat hippocampal neurons | 149 | |
| Heparan sulfate proteoglycans (Glypican 1) | SH-SY5Y neuroblastoma cells | 136 |
ApoE, apolipoprotein E.
In addition to the various lipid components, APP itself is expressed in lipid rafts (129–137). The Aβ-bearing C-terminal fragment produced by β-secretase (134, 138), the APP N-terminal fragment (139), and Aβs (129, 140, 141) have been identified in lipid rafts of cultured cells and mammalian brains. α-Secretase (135, 142–144), β-secretase (BACE1, Asp-2, memapsin-1) (21, 141, 143, 145, 146), and PSEN1(γ-secretase) (129, 133, 134, 138, 147), apoE, and tau are also known to be present in lipid rafts (141). Lipid rafts also express plasmin (148, 149) and glypican 1 (136). All these components may potentially contribute to the pathogenic mechanisms of AD, either by the formation of amyloid aggregates or by participating in the signaling pathways leading to apoptosis.
APP processing and lipid rafts
Lipid rafts have been implicated as sites for amyloidogenic processing of APP. APP present in the inside and outside of raft clusters seems to be cleaved by β-secretase and α-secretase, respectively (132, 137). The pathology of AD is closely correlated with the processing of APP and is sensitive to membrane alterations in levels of cholesterol and gangliosides. The cleavage by secretases occurs predominantly in post-Golgi secretory and endocytic compartments and is influenced by cholesterol, indicating a role of the membrane lipids in proteolytic processing of APPβ.
BACE1 (Asp 2) is a membrane-bound protease and is considered to be the major β-secretase that cleaves APP in the trans-Golgi network, an initial step in the pathogenesis of AD. Increasing evidence points to a close connection between cholesterol homeostasis and APP processing and Aβ production. Although cholesterol, gangliosides, BACE1, and γ-secretase, as well as Aβ, are supposed to be localized to rafts, there might be a yet-unknown biological function underlying these connections.
Grimm et al. (21) measured membrane fluidity of isolated DRM fractions from membrane preparations by anisotropy determination, and determined the cholesterol content of these fractions by a coupled enzymatic assay. They found that membrane fluidity is changed in embryonic PSEN1/PSEN2−/− mouse fibroblasts, along with altered cholesterol content in the DRM fraction of these cells. The ganglioside levels are enhanced in the absence of PSEN1. Sawamura et al. (41) reported that the levels of insoluble Aβ1-42 in the low-density DRMs were markedly increased in transgenic mice expressing mutant PSEN2 (N141I), compared with transgenic mice expressing wild-type PSEN2. In this case, the level of sphingomyelin decreased significantly, but not the level of gangliosides. Yamamoto et al. (42) reported that the concentration of GM1 in DRMs of synaptosomes increased with age and that this increase was more pronounced in brains of knock-in mice homozygous for the human apoE ε4 allele, compared with those harboring two ε3 alleles. In this case, they suggested that aging and apoE4 expression cooperatively accelerated Aβ aggregation in the brain through an increase in the level of GM1 in particular microdomains of neuronal membranes. These results suggest that Aβ accumulation in these microdomains is an early event in the process of AD development (42).
Aβ dimers and oligomers in lipid rafts
Aβ dimers are reported to be present in lipid rafts at the time when memory impairment begins in the Tg2576 APP-overexpressing transgenic mouse model of AD (141). After dimeric Aβ began to accumulate in lipid rafts of the brain, apoE and then phosphorylated tau accumulated. Kim, Yi, and Ko (137) reported that Aβ oligomerization was accelerated by the application of lipid rafts isolated from ganglioside-rich C2C12 cells, and this was not observed with lipid rafts isolated from ganglioside-poor cells such as SK-N-MC and HeLa cells. Interestingly, lipid raft-induced Aβ oligomerization is inhibited in CHO-1K1 cells, which are defective with regard to ganglioside biosynthesis. This indicates that Aβ oligomerization requires gangliosides that are enriched in the lipid rafts. Early evidence indicated that fibrillary aggregates of Aβ are neurotoxic. Recent reports suggest, however, that the toxicity of Aβ and other amyloidogenic proteins lies not in the insoluble amyloid fibrils or larger aggregates but rather in soluble small oligomeric species, which suggests that a more important goal for AD therapy is to find compounds capable of blocking Aβ oligomerization (150). Aβ oligomers appear immediately after the incubation of Aβ with lipid rafts isolated from the brain tissues of rats. However, these oligomers are typically not converted into Aβ fibrils, even after 4 days of incubation (137). Recent studies suggest that Aβ aggregation also occurs in lipid rafts mediated by a cluster of GM1. One study examined the effects of representative compounds on Aβ aggregation and fibril destabilization in the presence of GM1-containing raft-like liposomes (96). However, Yu et al. (151) reported that methods for biochemical isolation of these microdomains may produce artifacts. When synthetic Aβ1-40 monomers were added to the brain fragment at a final concentration of 2.1 μM, followed by homogenization and isolation of lipid rafts by an established method, Aβ1-40 accumulated as oligomers in the lipid raft fraction. However, in the absence of a brain homogenate, synthetic Aβ1-40 did not accumulate in the lipid raft fraction. These results imply that exogenous Aβ can be associated with lipid rafts, and Aβs bound to rafts can form oligomers during the isolation of lipid rafts. In conclusion, the exclusive expression of these components suggests a critical and decisive role for lipid rafts in Aβ generation and processing in AD (113).
Interaction of Aβ with gangliosides in lipid rafts or membrane microdomains may play a critical role in the pathogenesis of AD
As indicated above, gangliosides are enriched in the lipid rafts of brains of AD patients (124). Molander-Melin et al. (124) reported the presence of gangliosides in DRMs isolated from 10 AD and 10 age-matched control brains. DRMs from the frontal and temporal cortices of AD brains contained a significantly higher concentration of gangliosides GM1 and GM2. The DRMs derived from temporal cortex of AD patients were depleted of cholesterol and constituted a lower proportion of wet tissue weight, compared with control subjects. An increased proportion of GM1 and GM2 in DRMs accelerates the formation of Aβ plaque at an early stage, which may gradually lead to membrane raft disruptions and thereby affect cellular functions. Increased levels of GM1 in DRMs, especially in the frontal cortex, may favor conversion of Aβ to the insoluble form and promote plaque formation. Although a significant loss of gangliosides was found in the AD brain, the reduction was not reflected in the ganglioside content of DRMs from AD brain, which instead showed significantly increased levels of GM1 and GM2. Although the data are yet to be confirmed, they indicate that the raft-associated gangliosides, especially GM1, may play a role in conversion of Aβ to the insoluble form to promote plaque formation, resulting in the progression of AD. The authors further speculated that DRM with a high density of GM2 is related to dendritiogenesis (152). Interaction of Aβ and GM2 is not excluded, although its binding is less tight than binding to GM1 (11, 54).
There have been several in vitro studies concerning the involvement of lipid raft-associated components in the pathogenesis of AD. Mizuno et al. (153) reported that a conformationally altered form of Aβ, which acts as a “seed” for amyloid fibril formation, may be generated in intracellular cholesterol-rich microdomains. Among major brain gangliosides (GM1, GD1a, GD1b, and GT1b), GM1-Aβ exhibits the strongest seeding potential, especially under β-sheet-forming conditions (72). Seeding formation is facilitated by cholesterol; Aβ binding to GM1 is markedly accelerated in a cholesterol-rich environment (154). Increases in intramembrane cholesterol content, which are likely to occur during aging, appear to be a risk factor for amyloid fibril formation (154). In addition, these investigators reported the involvement of lipid rafts as the interaction sites for soluble Aβ to form toxic Aβ aggregation (155). Lipid rafts containing ganglioside clusters may serve as a conformational catalyst or chaperones to generate a membrane-active form of Aβ with seeding ability rather than providing a specific binding site for the protein (155). Further studies indicate that aggregation of soluble Aβ is readily induced by its interaction with lipid raft-like model membranes composed of GM1-cholesterol-sphingomyelin (1:1:1) (73, 154), and that Aβ becomes neurotoxic upon incubation with these liposomes (156). After Aβ binds to raft-like membranes, the protein can translocate to the phosphatidylcholine membranes to which soluble Aβ does not bind (155). These findings suggest the relevance of GM1 and other gangliosides in AD pathology, for example, in inducing aggregation of soluble Aβ in a raft-associated process (155).
GAβ, found in brains exhibiting early pathological changes in AD plaques, has been suggested to accelerate amyloid fibril formation by acting as a seed, as described above (78). GAβ may be generated in the lipid rafts or lipid raft-like microdomains in neurons because of an increase in the local concentration of cholesterol, and then accelerates Aβ aggregation after its transport to the neuronal surface and/or shedding into the extracellular space (156). It may also be worth examining in future studies whether GAβ causes impairment of neuronal, particularly lipid raft-related, functions before extraneuronal Aβ deposition (157). Alternatively, GAβ itself can be noxious per se; it has been reported previously that disruption of membranes (55) and alteration of bilayer organization (53) can be induced by generation of GAβ on the membranes (Fig. 7).
Fig. 7.
Interactions of gangliosides and amyloid β-proteins in lipid rafts.
THERAPEUTIC POTENTIALS OF GANGLIOSIDES IN PATIENTS WITH AD
Neuronal progenitor cells (NPCs) are being considered for treatment of neurodegenerative diseases associated with β-amyloidosis in AD and DS (158). Transplantation of NPCs may replace lost neurons and restore brain functions in AD and AD/DS by reduction of neurotoxic amyloid and by their antioxidant properties. The stimulatory effect of Aβ on neurogenesis has recently been postulated. Zou et al. (159) showed a novel function of monomeric Aβ1-40, which is the major species found in physiological fluid, as a natural antioxidant molecule that prevents neuronal death caused by transition metal-induced oxidative damage. Treatment of hippocampal neural stem cells with Aβ peptide induced an increase in the number of newborn neurons with no change in the rate of cell death or proliferation (160), suggesting that the Aβ peptide could act on neuronal progenitors and promote their differentiation into neurons (161, 162). Taken together, neural cell replacement therapy using NPCs in AD may be another excellent strategy for removal of Aβ and oxidative damage (162, 163).
Neural and glial cells organizing the central nervous system (CNS) are generated from common NPCs during neural development. Very different gangliosides are expressed in adult CNS and NPCs (4, 5, 164). In fact, NPCs express several specific antigens, including several b-series gangliosides, such as GD3, 9-O-acetyl GD3, GT1b, and GQ1b, and stage-specific embryonic antigens, such as GQ1bα and HNK-1. Gangliosides are particularly abundant in the nervous system, and their species and amounts undergo dramatic changes during development in response to environmental cues (3, 5). There has been increasing interest in promoting resident NPCs in adults for neural regeneration. It would be interesting to examine NPC-associated gangliosides for their role in adult neurogenesis and neuroreplacement therapy in AD. Clearly, this represents a growing and promising area for AD therapy.
Beneficial effects of GM1 have been documented in the treatment of stroke and spinal cord injuries, particularly when the treatment is initiated within a few hours of the acute event (165). Continuous intraventricular infusion of GM1 has been shown to have a modest beneficial effect in early-onset AD (165–168). The optimal GM1 dose varied between 20 and 30 mg/24 h. The patients became more active, felt safer, and had improved reading comprehension and better feeling for language. NGFs and GM1 have been used to inhibit progression of AD, but this treatment is still at an experimental stage, as are efforts to prevent the formation of amyloid plaques (168).
Matsuoka et al. (169) reported the effect of peripherally administered GM1 on the level of Aβs in the brain using mice bearing two human mutant genes, an exon 9 deletion in PSEN1 and the Swedish APP double mutation. GM1-treated mice showed a substantial decrease in aggregated Aβ1-40 and Aβ1-42 in the brain. This observation suggests that in vivo GM1 administration may reduce or prevent brain amyloidosis. The anti-amyloidogenic effect was attributed to a “peripheral sink” effect, i.e., that GM1-bound Aβ alters central/peripheral dynamics, drawing Aβ out of the brain. In addition, GM1-bound Aβ in the blood is no longer available to cross the blood-brain barrier (BBB) and incorporate into plaques centrally. Thus, peripheral administration of GM1 may be effective in reducing amyloid aggregation in AD. A potential difficulty, however, in tapping the neuroprotective effects of GM1 is the low level of gangliosides that can enter into the brain. In this regard, Saulino and Schengrund (170) reported that only ∼1% of peripherally administered GM1 crosses the BBB to enter the brain. One way around this problem is the use of a more-permanent lyso derivative of GM1, II3-Neu-5-AcGgOse4-2-d-erythro-1,3-dihydroxy-2-dichloroacetylamide-4-trans-octadecene (LIGA 20). For example, LIGA 20 given after cortical thrombosis has been reported to reduce infarct size and associated cognitive deficits (171, 172). Oral administration of LIGA 20 protects neurons against glutamate-induced neuronal death in rats without producing the side effects typical of glutamate receptor antagonists (173) and in mice subject to kainate-induced seizures and apoptosis (174). The level of LIGA 20 in brains of treated animals can reach a significantly higher level than can GM1; moreover, animals treated with LIGA 20 showed no apparent biochemical or behavioral adverse effects (173, 175), suggesting that this compound may be useful as a neuroprotective agent in neuronal cell death in AD.
Exogenous GM1 treatment has also been used to treat symptoms of Parkinson's disease, in both human and animal models. GM1 protects against nigrostriatal toxicity and associated motor symptoms induced by 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine in mice and monkeys (171, 176–178). GM1 treatment restored cognitive and motor function and prevented decline in function in a 90 week trial in monkeys (179). In a double-blind, placebo-controlled study, Parkinson's patients improved significantly after 16 weeks of GM1 treatment, compared with both the placebo group and their own initial baselines (180). In addition to GM1, Schneider et al. (181) reported potential neuroprotective and neurorestorative roles for the synthetic ceramide analog l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (l-PDMP) in mouse models of Parkinsonism. In contrast to the d-isomer, l-PDMP treatment caused an increase in brain GM1 levels in Parkinson models. Thus, administration of l-PDMP as a means to elevate endogenous brain GM1 levels may hold promise as a potential neuroprotective or neurorestorative therapeutic strategy for Parkinson's disease. Importantly, l-PDMP induces an increase in endogenous GM1. Odaka et al. (182) reported that GM1 may act as an immunogen in some patients who develop Guillain-Barré syndrome following ganglioside therapy. Therefore, l-PDMP might be useful as pivotal neuroprotective therapy in AD.
Yanagisawa and Ihara (81) have reported that Aβ adopts an altered conformation through binding to GM1 in brain, which subsequently facilitates the assembly of soluble Aβ by acting as an endogenous seed, GAβ. For this reason, it would be advantageous to target endogenous seeds as a therapeutic strategy. In fact, Hayashi et al. (156) developed a novel monoclonal antibody raised against purified GAβ from AD brain. Notably, this antibody, 4396C, substantially inhibits Aβ assembly in vitro, specifically binds to GAβ and Aβ, and importantly, does not bind to either monomeric Aβ or amyloid fibrils deposited as plaques. In addition, this antibody potently inhibits the assembly of Aβ1-40 and Aβ1-42 in vitro. Furthermore, Yamamoto et al. (157) attempted peripheral administration of Fab fragments of 4396C to transgenic mice expressing a mutant APP gene, resulting in markedly suppressed Aβ deposition in the brain. These findings add a new dimension to the development of novel therapeutic strategies by targeting seeded Aβ in the brain, which selectively inhibits the initial step of the pathological process of AD (52, 79).
Recent studies suggest that Aβ aggregation occurs in lipid rafts mediated by a cluster of GM1 (96). Interestingly, rifampicin and nordihydroguaiaretic acid have been shown to inhibit binding of Aβ to GM1 liposomes by competitively binding to the membranes and/or direct interaction with Aβ in solution, thus at least partly preventing fibrils from forming. These small compounds can therefore be utilized for therapeutics. With the finding that GAβ has a conformation distinct from that of soluble Aβ, it may be possible to develop a novel therapeutic strategy to specifically inhibit the initiation of oligomerization-polymerization of Aβ in the brain (156, 157).
CONCLUDING REMARKS
Gangliosides are known to play a neuroprotective role in in vivo and in vitro models of neuronal injury. The mechanisms of the neuroprotective effect of GM1 and related gangliosides, however, are still obscure. Several lines of evidence document the bifunctional roles of gangliosides in in vivo and in vitro studies. Because gangliosides are enriched in neuronal membranes and bind to β-amyloid and APP, they are probably involved in the conformational changes in these molecules that are a hallmark of the pathogenesis of AD. Recent studies have demonstrated that an increase in the level of GM1 results in the aggregation of Aβ in vitro and that AD brains expressed unusual deposits, such as the GAβ complexes. These observations indicate that neuronal gangliosides are involved in the accumulation of circulating Aβ to form complexes that are expressed in AD brain. Because it has been demonstrated that GM1 possesses neurotrophic properties, administration of GM1 may exert beneficial effects in AD. Additionally, GM1 infusions may be useful for the sequestration of excess Aβ in AD patients to alleviate their toxic effects. Future studies should be directed toward the therapeutic potentials of gangliosides in AD.
Acknowledgments
The authors acknowledge the assistance of Drs. Makoto Yanagisawa and Alex Chiu during the course of this investigation and the expert editorial help of Dr. Rhea Markowitz.
Abbreviations
Aβ, amyloid β-protein
AD, Alzheimer's disease
apoE, apolipoprotein E
APP, amyloid precursor protein
BBB, blood-brain barrier
CNS, central nervous system
CSF, cerebrospinal fluid
Ctxb, cholera toxin B-subunit
DAT, dementia of the Alzheimer type
DRM, detergent-resistant membrane
DS, Down syndrome
FL-Aβ, fluorescein-labeled Aβ
HPTLC, high-performance thin-layer chromatography
GAβ, GM1-bound Aβ complex
GD3S, GD3 synthase
GEM, glycolipid-enriched membrane
GPI, glycosylphosphatidylinositol
GSL, glycosphingolipid
LIGA 20, II3-Neu-5-AcGgOse4-2-d-erythro-1,3-dihydroxy-2-dichloroacetylamide-4-trans-octadecene
l-PDMP, l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
NGF, nerve growth factor
NPC, neuronal progenitor cell
PSEN, presenilin
SP, senile plaque
SPR, surface plasmon resonance
ST6Gal I, β-galactosidase α2,6-sialytransferase
Published, JLR Papers in Press, March 11, 2008.
Footnotes
This study was supported by National Institutes of Health Grants AG-027199, NS-026994, and NS-011853 to R.K.Y. and AG-022439 to M.P.M.
This article is dedicated to the memory of Dr. Herbert E. Carter.
References
- 1.Hakomori S. 2003. Structure, organization, and function of glycosphingolipids in membrane. Curr. Opin. Hematol. 10 16–24. [DOI] [PubMed] [Google Scholar]
- 2.Yu, R. K., M. Yanagisawa, and T. Ariga. 2007. Glycosphingolipid structures. In Comprehensive Glycoscience. Elsevier, Oxford, UK. 73–122.
- 3.Yu R. K. 1994. Development regulation of ganglioside metabolism. Prog. Brain Res. 101 31–44. [DOI] [PubMed] [Google Scholar]
- 4.Yanagisawa M., and R. K. Yu. 2007. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 17 57R–74R. [DOI] [PubMed] [Google Scholar]
- 5.Ngamukote S., M. Yanagisawa, T. Ariga, S. Ando, and R. K. Yu. 2007. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 103 2327–2341. [DOI] [PubMed] [Google Scholar]
- 6.Yu R. K., S. Usuki, and T. Ariga. 2006. Ganglioside molecular mimicry and its pathological roles in Guillain-Barrè syndrome and related diseases. Infect. Immun. 74 6517–6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledeen R., and G. Wu. 2007. GM1 in the nuclear envelope regulates nuclear calcium through association with a nuclear sodium-calcium exchanger. J. Neurochem. 103 (Suppl.): 126–134. [DOI] [PubMed] [Google Scholar]
- 8.Parton R. G. 1994. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42 155–166. [DOI] [PubMed] [Google Scholar]
- 9.Simons K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1 31–39. [DOI] [PubMed] [Google Scholar]
- 10.Yang L. J., C. B. Zeller, N. L. Shaper, M. Kiso, A. Hasegawa, R. E. Shapiro, and R. L. Schnaar. 1996. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc. Natl. Acad. Sci. USA. 93 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariga T., K. Kobayashi, A. Hasegawa, M. Kiso, H. Ishida, and T. Miyatake. 2001. Characterization of high-affinity binding between gangliosides and amyloid β-protein. Arch. Biochem. Biophys. 388 225–230. [DOI] [PubMed] [Google Scholar]
- 12.Hakomori S., and Y. Igarashi. 1995. Functional role of glycosphingolipids in cell recognition and signaling. J. Biochem. 118 1091–1103. [DOI] [PubMed] [Google Scholar]
- 13.Ledeen R. W., G. Wu, Z. H. Lu, D. Kozireski-Chuback, and Y. Fang. 1998. The role of GM1 and other gangliosides in neuronal differentiation. Overview and new finding. Ann. N. Y. Acad. Sci. 845 161–175. [DOI] [PubMed] [Google Scholar]
- 14.Hadjiconstantinou M., and N. H. Neff. 1998. GM1 ganglioside: in vivo and in vitro trophic actions on central neurotransmitter systems. J. Neurochem. 70 1335–1345. [DOI] [PubMed] [Google Scholar]
- 15.Sokolova T. V., I. O. Zakharova, V. V. Furaev, M. P. Rychkova, and N. F. Avrova. 2007. Neuroprotective effect of ganglioside GM1 on the cytotoxic action of hydrogen peroxide and amyloid β-peptide in PC12 cells. Neurochem. Res. 32 1302–1313. [DOI] [PubMed] [Google Scholar]
- 16.Terzi E., G. Holzemann, and J. Seelig. 1995. Self-association of β-amyloid peptide (1-40) in solution and binding to lipid membranes. J. Mol. Biol. 252 633–642. [DOI] [PubMed] [Google Scholar]
- 17.Selkoe D. J. 2002. Alzheimer's disease is a synaptic failure. Science. 298 789–791. [DOI] [PubMed] [Google Scholar]
- 18.Bellucci A., M. C. Rosi, C. Grossi, A. Fiorentini, I. Luccarini, and F. Casamenti. 2007. Abnormal processing of tau in the brain of aged TgCRND8 mice. Neurobiol. Dis. 27 328–338. [DOI] [PubMed] [Google Scholar]
- 19.Turner P. R., K. O'Connor, W. P. Tate, and W. C. Abraham. 2003. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 70 1–32. [DOI] [PubMed] [Google Scholar]
- 20.Mutoh T., Y. Hirabayashi, T. Mihara, M. Ueda, H. Koga, A. Ueda, T. Kokura, and H. Yamamoto. 2006. Role of glycosphingolipids and therapeutic perspectives on Alzheimer's disease. CNS Neurol. Disord. Drug Targets. 5 375–380. [DOI] [PubMed] [Google Scholar]
- 21.Grimm M. O., J. A. Tschape, H. S. Grimm, E. G. Zinser, and T. Hartmann. 2006. Altered membrane fluidity and lipid raft composition in presenilin-deficient cells. Acta Neurol. Scand. Suppl. 185 27–32. [DOI] [PubMed] [Google Scholar]
- 22.Kalanj-Bognar S. 2006. Ganglioside catabolism is altered in fibroblasts and leukocytes from Alzheimer's disease patients. Neurobiol. Aging. 27 1354–1356. [DOI] [PubMed] [Google Scholar]
- 23.Svennerholm L., and C. G. Gottfries. 1994. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). J. Neurochem. 62 1039–1047. [DOI] [PubMed] [Google Scholar]
- 24.Kalanj S., I. Kracun, H. Rosner, and C. Cosovic. 1991. Regional distribution of brain gangliosides in Alzheimer's disease. Neurol. Croat. 40 269–281. [PubMed] [Google Scholar]
- 25.Brooksbank B. W., and J. McGovern. 1989. Gangliosides in the brain in adult Down's syndrome and Alzheimer's disease. Mol. Chem. Neuropathol. 11 143–156. [DOI] [PubMed] [Google Scholar]
- 26.Crino P. B., M. D. Ullman, B. A. Vogt, E. D. Bird, and L. Volicer. 1989. Brain gangliosides in dementia of the Alzheimer type. Arch. Neurol. 46 398–401. [DOI] [PubMed] [Google Scholar]
- 27.Kracun I., S. Kalanj, C. Cosovic, and J. Talan-Hranilovic. 1990. Brain gangliosides in Alzheimer's disease. J. Hirnforsch. 31 789–793. [PubMed] [Google Scholar]
- 28.Kracun I., H. Rosner, V. Drnovsek, M. Heffer-Lauc, C. Cosovic, and G. Lauc. 1991. Human brain gangliosides in development, aging and disease. Int. J. Dev. Biol. 35 289–295. [PubMed] [Google Scholar]
- 29.Kracun I., S. Kalanj, J. Talan-Hranilovic, and C. Cosovic. 1992. Cortical distribution of gangliosides in Alzheimer's disease. Neurochem. Int. 20 433–438. [DOI] [PubMed] [Google Scholar]
- 30.Hirabayashi Y., T. Nakao, F. Irie, V. P. Whittaker, K. Kon, and S. Ando. 1992. Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. J. Biol. Chem. 267 12973–12978. [PubMed] [Google Scholar]
- 31.Ando S., Y. Hirabayashi, K. Kon, F. Inagaki, S. Tate, and V. P. Whittaker. 1992. A trisialoganglioside containing a sialyl α2-6 N-acetylgalactosamine residue is a cholinergic-specific antigen, Chol-1α. J. Biochem. 111 287–290. [DOI] [PubMed] [Google Scholar]
- 32.Derrington E. A., and E. Borroni. 1990. The developmental expression of the cholinergic-specific antigen Chol-1 in the central and peripheral nervous system of the rat. Brain Res. Dev. Brain Res. 52 131–140. [DOI] [PubMed] [Google Scholar]
- 33.Blennow K., P. Davidsson, A. Wallin, P. Fredman, C. G. Gottfries, J. E. Mansson, and L. Svennerholm. 1992. Differences in cerebrospinal fluid gangliosides between “probable Alzheimer's disease” and normal aging. Aging (Milano). 4 301–306. [PubMed] [Google Scholar]
- 34.Nishinaka T., D. Iwata, S. Shimada, K. Kosaka, and Y. Suzuki. 1993. Anti-ganglioside GD1a monoclonal antibody recognizes senile plaques in the brains of patients with Alzheimer-type dementia. Neurosci. Res. 17 171–176. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H., K. Hirokawa, S. Ando, and K. Obata. 1991. Immunohistological study on brains of Alzheimer's disease using antibodies to fetal antigens, C-series gangliosides and microtubule-associated protein 5. Acta Neuropathol. (Berl.). 81 626–631. [DOI] [PubMed] [Google Scholar]
- 36.Tooyama I., T. Yamada, S. U. Kim, and P. L. McGeer. 1992. Immunohistochemical study of A2B5-positive ganglioside in postmortem human brain tissue of Alzheimer disease, amyotrophic lateral sclerosis, progressive supranuclear palsy and control cases. Neurosci. Lett. 136 91–94. [DOI] [PubMed] [Google Scholar]
- 37.Majocha R. E., F. B. Jungalwala, A. Rodenrys, and C. A. Marotta. 1989. Monoclonal antibody to embryonic CNS antigen A2B5 provides evidence for the involvement of membrane components at sites of Alzheimer degeneration and detects sulfatides as well as gangliosides. J. Neurochem. 53 953–961. [DOI] [PubMed] [Google Scholar]
- 38.Kasai N., and R. K. Yu. 1983. The monoclonal antibody A2B5 is specific for ganglioside GQ1c. Brain Res. 277 155–158. [DOI] [PubMed] [Google Scholar]
- 39.Barrier L., S. Ingrand, M. Damjanac, A. Rioux Bilan, J. Hugon, and G. Page. 2006. Genotype-related changes of ganglioside composition in brain regions of transgenic mouse models of Alzheimer's disease. Neurobiol. Aging. 28 1863–1872. [DOI] [PubMed] [Google Scholar]
- 40.Bernardo, A., F. E. Harrison, M. McCord, J. Zhao, A. Bruchey, S. S. Davies, L. Jackson Roberts II, P. M. Mathews, Y. Matsuoka, T. Ariga, et al. Elimination of GD3 synthase improves memory and reduces amyloid-β plaque load in transgenic mice. Neurobiol. Aging. Epub ahead of print. February 5, 2008; doi:10.1016/j.neurobiolaging.12.022.2007. [DOI] [PubMed]
- 41.Sawamura N., M. Morishima-Kawashima, H. Waki, K. Kobayashi, T. Kuramochi, M. P. Frosch, K. Ding, M. Ito, T. W. Kim, R. E. Tanzi, et al. 2000. Mutant presenilin 2 transgenic mice. A large increase in the levels of Aβ42 is presumably associated with the low density membrane domain that contains decreased levels of glycerophospholipids and sphingomyelin. J. Biol. Chem. 275 27901–27908. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto N., U. Igbavboa, Y. Shimada, Y. Ohno-Iwashita, M. Kobayashi, W. G. Wood, S. C. Fujita, and K. Yanagisawa. 2004. Accelerated Aβ aggregation in the presence of GM1-ganglioside-accumulated synaptosomes of aged apoE4-knock-in mouse brain. FEBS Lett. 569 135–139. [DOI] [PubMed] [Google Scholar]
- 43.Pitto M., F. Raimondo, C. Zoia, L. Brighina, C. Ferrarese, and M. Masserini. 2005. Enhanced GM1 ganglioside catabolism in cultured fibroblasts from Alzheimer patients. Neurobiol. Aging. 26 833–838. [DOI] [PubMed] [Google Scholar]
- 44.Kalanj-Bognar S., T. Rundek, I. Furac, V. Demarin, and C. Cosovic. 2002. Leukocyte lysosomal enzymes in Alzheimer's disease and Down's syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 57 B16–B21. [DOI] [PubMed] [Google Scholar]
- 45.Maguire T. M., and K. C. Breen. 1995. A decrease in neural sialyltransferase activity in Alzheimer's disease. Dementia. 6 185–190. [DOI] [PubMed] [Google Scholar]
- 46.Maguire T. M., A. M. Gillian, D. O'Mahony, C. M. Coughlan, A. Dennihan, and K. C. Breen. 1994. A decrease in serum sialyltransferase levels in Alzheimer's disease. Neurobiol. Aging. 15 99–102. [DOI] [PubMed] [Google Scholar]
- 47.Cataldo A. M., J. L. Barnett, D. M. Mann, and R. A. Nixon. 1996. Colocalization of lysosomal hydrolase and β-amyloid in diffuse plaques of the cerebellum and striatum in Alzheimer's disease and Down's syndrome. J. Neuropathol. Exp. Neurol. 55 704–715. [DOI] [PubMed] [Google Scholar]
- 48.Lehmann H. C., P. H. Lopez, G. Zhang, T. Ngyuen, J. Zhang, B. C. Kieseier, S. Mori, and K. A. Sheikh. 2007. Passive immunization with anti-ganglioside antibodies directly inhibits axon regeneration in an animal model. J. Neurosci. 27 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuki N., and T. Ariga. 1997. Antibodies to fucogangliosides in neurological diseases. J. Neurol. Sci. 150 81–84. [DOI] [PubMed] [Google Scholar]
- 50.Chapman J., B. A. Sela, E. Wertman, and D. M. Michaelson. 1988. Antibodies to ganglioside GM1 in patients with Alzheimer's disease. Neurosci. Lett. 86 235–240. [DOI] [PubMed] [Google Scholar]
- 51.Chapman J., O. Bachar, A. D. Korczyn, E. Wertman, and D. M. Michaelson. 1988. Antibodies to cholinergic neurons in Alzheimer disease. J. Neurochem. 51 479–485. [DOI] [PubMed] [Google Scholar]
- 52.Yanagisawa K. 2005. GM1 ganglioside and the seeding of amyloid in Alzheimer's disease: endogenous seed for Alzheimer amyloid. Neuroscientist. 11 250–260. [DOI] [PubMed] [Google Scholar]
- 53.Matsuzaki K., and C. Horikiri. 1999. Interactions of amyloid β-peptide (1-40) with ganglioside-containing membranes. Biochemistry. 38 4137–4142. [DOI] [PubMed] [Google Scholar]
- 54.Choo-Smith L. P., W. Garzon-Rodriguez, C. G. Glabe, and W. K. Surewicz. 1997. Acceleration of amyloid fibril formation by specific binding of Aβ-(1-40) peptide to ganglioside-containing membrane vesicles. J. Biol. Chem. 272 22987–22990. [DOI] [PubMed] [Google Scholar]
- 55.McLaurin J., and A. Chakrabartty. 1996. Membrane disruption by Alzheimer β-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J. Biol. Chem. 271 26482–26489. [DOI] [PubMed] [Google Scholar]
- 56.McLaurin J., T. Franklin, P. E. Fraser, and A. Chakrabartty. 1998. Structural transitions associated with the interaction of Alzheimer β-amyloid peptides with gangliosides. J. Biol. Chem. 273 4506–4515. [DOI] [PubMed] [Google Scholar]
- 57.Atwood C. S., R. D. Moir, X. Huang, R. C. Scarpa, N. M. Bacarra, D. M. Romano, M. A. Hartshorn, R. E. Tanzi, and A. I. Bush. 1998. Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 273 12817–12826. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y. R., H. B. Huang, C. L. Chyan, M. S. Shiao, T. H. Lin, and Y. C. Chen. 2006. The effect of Aβ conformation on the metal affinity and aggregation mechanism studied by circular dichroism spectroscopy. J. Biochem. 139 733–740. [DOI] [PubMed] [Google Scholar]
- 59.Choo-Smith L. P., and W. K. Surewicz. 1997. The interaction between Alzheimer amyloid β(1-40) peptide and ganglioside GM1-containing membranes. FEBS Lett. 402 95–98. [DOI] [PubMed] [Google Scholar]
- 60.Matsuzaki K. 2007. Physicochemical interactions of amyloid β-peptide with lipid bilayers. Biochim. Biophys. Acta. 1768 1935–1942. [DOI] [PubMed] [Google Scholar]
- 61.Chi E. Y., S. L. Frey, and K. Y. Lee. 2007. Ganglioside G(M1)-mediated amyloid-β fibrillogenesis and membrane disruption. Biochemistry. 46 1913–1924. [DOI] [PubMed] [Google Scholar]
- 62.Kurganov B., M. Doh, and N. Arispe. 2004. Aggregation of liposomes induced by the toxic peptides Alzheimer's Aβs, human amylin and prion (106-126): facilitation by membrane-bound GM1 ganglioside. Peptides. 25 217–232. [DOI] [PubMed] [Google Scholar]
- 63.Valdes-Gonzalez T., J. Inagawa, and T. Ido. 2001. Neuropeptides interact with glycolipid receptors: a surface plasmon resonance study. Peptides. 22 1099–1106. [DOI] [PubMed] [Google Scholar]
- 64.Inaba S., T. Okada, T. Konakahara, and M. Kodaka. 2005. Specific binding of amyloid-β-protein to IMR-32 neuroblastoma cell membrane. J. Pept. Res. 65 485–490. [DOI] [PubMed] [Google Scholar]
- 65.Mandal P. K., and J. W. Pettegrew. 2004. Alzheimer's disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with Aβ(1-40) peptide in a membrane mimic environment. Neurochem. Res. 29 447–453. [DOI] [PubMed] [Google Scholar]
- 66.Williamson M. P., Y. Suzuki, N. T. Bourne, and T. Asakura. 2006. Binding of amyloid β-peptide to ganglioside micelles is dependent on histidine-13. Biochem. J. 397 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chauhan A., I. Ray, and V. P. Chauhan. 2000. Interaction of amyloid β-protein with anionic phospholipids: possible involvement of Lys28 and C-terminus aliphatic amino acids. Neurochem. Res. 25 423–429. [DOI] [PubMed] [Google Scholar]
- 68.Lee J. P., E. R. Stimson, J. R. Ghilardi, P. W. Mantyh, Y. A. Lu, A. M. Felix, W. Llanos, A. Behbin, M. Cummings, M. Van Criekinge, et al. 1995. 1H NMR of Aβ amyloid peptide congeners in water solution. Conformational changes correlate with plaque competence. Biochemistry. 34 5191–5200. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, H., J. Ding, W. Tian, L. Wang, L. Huang, Y. Ruan, T. Lu, Y. Sha, and D. Zhang. Ganglioside GM1 binding the N-terminus of amyloid precursor protein. Neurobiol. Aging. Epub ahead of print. December 10, 2007; doi:10.1016/j.neurobiolaging.11.013.2007. [DOI] [PubMed]
- 70.Vigo-Pelfrey C., D. Lee, P. Keim, I. Lieberburg, and D. B. Schenk. 1993. Characterization of β-amyloid peptide from human cerebrospinal fluid. J. Neurochem. 61 1965–1968. [DOI] [PubMed] [Google Scholar]
- 71.Zou K., D. Kim, A. Kakio, K. Byun, J. S. Gong, J. Kim, M. Kim, N. Sawamura, S. Nishimoto, K. Matsuzaki, et al. 2003. Amyloid β-protein (Aβ)1-40 protects neurons from damage induced by Aβ1-42 in culture and in rat brain. J. Neurochem. 87 609–619. [DOI] [PubMed] [Google Scholar]
- 72.Kakio A., S. Nishimoto, K. Yanagisawa, Y. Kozutsumi, and K. Matsuzaki. 2002. Interactions of amyloid β-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry. 41 7385–7390. [DOI] [PubMed] [Google Scholar]
- 73.Kakio A., Y. Yano, D. Takai, Y. Kuroda, O. Matsumoto, Y. Kozutsumi, and K. Matsuzaki. 2004. Interaction between amyloid β-protein aggregates and membranes. J. Pept. Sci. 10 612–621. [DOI] [PubMed] [Google Scholar]
- 74.Okada T., M. Wakabayashi, K. Ikeda. And, and K. Matsuzaki. 2007. Formation of toxic fibrils of Alzheimer's β-protein-(1–40) by monosialoganglioside GM1, a neuronal membrane component. J. Mol. Biol. 371 481–489. [DOI] [PubMed] [Google Scholar]
- 75.Yanagisawa K. 2003. Cholesterol and Aβ aggregation. Pharmacopsychiatry. 36 (Suppl.): 127–129. [DOI] [PubMed] [Google Scholar]
- 76.Yanagisawa K., and K. Matsuzaki. 2002. Cholesterol-dependent aggregation of amyloid beta-protein. Ann. N. Y. Acad. Sci. 977 384–386. [DOI] [PubMed] [Google Scholar]
- 77.Yanagisawa K., A. Odaka, N. Suzuki, and Y. Ihara. 1995. GM1 ganglioside-bound amyloid β-protein (Aβ): a possible form of preamyloid in Alzheimer's disease. Nat. Med. 1 1062–1066. [DOI] [PubMed] [Google Scholar]
- 78.Yanagisawa K. 2007. Role of gangliosides in Alzheimer's disease. Biochim. Biophys. Acta. 1768 1943–1951. [DOI] [PubMed] [Google Scholar]
- 79.Kimura N., and K. Yanagisawa. 2007. Endosomal accumulation of GM1 ganglioside-bound amyloid β-protein in neurons of aged monkey brains. Neuroreport. 18 1669–1673. [DOI] [PubMed] [Google Scholar]
- 80.Yanagisawa K., and Y. Ihara. 1998. GM1 ganglioside-bound amyloid β-protein in Alzheimer's disease brain. Neurobiol. Aging. 19 (Suppl.): 65–67. [DOI] [PubMed] [Google Scholar]
- 81.Yamamoto N., K. Matsuzaki, and K. Yanagisawa. 2005. Cross-seeding of wild-type and hereditary variant-type amyloid β-proteins in the presence of gangliosides. J. Neurochem. 95 1167–1176. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto N., W. E. Van Nostrand, and K. Yanagisawa. 2006. Further evidence of local ganglioside-dependent amyloid β-protein assembly in brain. Neuroreport. 17 1735–1737. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto N., K. Hasegawa, K. Matsuzaki, H. Naiki, and K. Yanagisawa. 2004. Environment- and mutation-dependent aggregation behavior of Alzheimer amyloid β-protein. J. Neurochem. 90 62–69. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto N., E. Matsubara, S. Maeda, H. Minagawa, A. Takashima, W. Maruyama, M. Michikawa, and K. Yanagisawa. 2007. A ganglioside-induced toxic soluble Aβ assembly. Its enhanced formation from Aβ bearing the Arctic mutation. J. Biol. Chem. 282 2646–2655. [DOI] [PubMed] [Google Scholar]
- 85.Zha Q., Y. Ruan, T. Hartmann, K. Beyreuther, and D. Zhang. 2004. GM1 ganglioside regulates the proteolysis of amyloid precursor protein. Mol. Psychiatry. 9 946–952. [DOI] [PubMed] [Google Scholar]
- 86.Tamboli I. Y., K. Prager, E. Barth, M. Heneka, K. Sandhoff, and J. Walter. 2005. Inhibition of glycosphingolipid biosynthesis reduces secretion of the β-amyloid precursor protein and amyloid β-peptide. J. Biol. Chem. 280 28110–28117. [DOI] [PubMed] [Google Scholar]
- 87.Sawamura N., M. Ko, W. Yu, K. Zou, K. Hanada, T. Suzuki, J. S. Gong, K. Yanagisawa, and M. Michikawa. 2004. Modulation of amyloid precursor protein cleavage by cellular sphingolipids. J. Biol. Chem. 279 11984–11991. [DOI] [PubMed] [Google Scholar]
- 88.Kitazume S., Y. Tachida, R. Oka, K. Shirotani, T. C. Saido, and Y. Hashimoto. 2001. Alzheimer's β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc. Natl. Acad. Sci. USA. 98 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kitazume S., K. Nakagawa, R. Oka, Y. Tachida, K. Ogawa, Y. Luo, M. Citron, H. Shitara, C. Taya, H. Yonekawa, et al. 2005. In vivo cleavage of α2,6-sialyltransferase by Alzheimer β-secretase. J. Biol. Chem. 280 8589–8595. [DOI] [PubMed] [Google Scholar]
- 90.Nakagawa K., S. Kitazume, R. Oka, K. Maruyama, T. C. Saido, Y. Sato, T. Endo, and Y. Hashimoto. 2006. Sialylation enhances the secretion of neurotoxin amyloid-β peptides. J. Neurochem. 96 924–933. [DOI] [PubMed] [Google Scholar]
- 91.Greene L. A., and A. S. Tischler. 1976. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA. 73 2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dichter M. A., A. S. Tischler, and L. A. Greene. 1977. Nerve growth factor-induced increase in electrical excitability and acetylcholine sensitivity of a rat pheochromocytoma cell line. Nature. 268 501–504. [DOI] [PubMed] [Google Scholar]
- 93.Wang S. S., D. L. Rymer, and T. A. Good. 2001. Reduction in cholesterol and sialic acid content protects cells from the toxic effects of β-amyloid peptides. J. Biol. Chem. 276 42027–42034. [DOI] [PubMed] [Google Scholar]
- 94.Wakabayashi M., and K. Matsuzaki. 2007. Formation of amyloids by Aβ-(1-42) on NGF-differentiated PC12 cells: roles of gangliosides and cholesterol. J. Mol. Biol. 371 924–933. [DOI] [PubMed] [Google Scholar]
- 95.Matsuzaki K., T. Noguch, M. Wakabayashi, K. Ikeda, T. Okada, Y. Ohashi, M. Hoshino, and H. Naiki. 2007. Inhibitors of amyloid β-protein aggregation mediated by GM1-containing raft-like membranes. Biochim. Biophys. Acta. 1768 122–130. [DOI] [PubMed] [Google Scholar]
- 96.Yuyama K., N. Yamamoto, and K. Yanagisawa. 2006. Chloroquine-induced endocytic pathway abnormalities: cellular model of GM1 ganglioside-induced Aβ fibrillogenesis in Alzheimer's disease. FEBS Lett. 580 6972–6976. [DOI] [PubMed] [Google Scholar]
- 97.Wakabayashi M., T. Okada, Y. Kozutsumi, and K. Matsuzaki. 2005. GM1 ganglioside-mediated accumulation of amyloid β-protein on cell membranes. Biochem. Biophys. Res. Commun. 328 1019–1023. [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto N., Y. Fukata, M. Fukata, and K. Yanagisawa. 2007. GM1- ganglioside-induced Aβ assembly on synaptic membranes of cultured neurons. Biochim. Biophys. Acta. 1768 1128–1137. [DOI] [PubMed] [Google Scholar]
- 99.Yanagisawa M., T. Ariga, and R. K. Yu. 2006. Cholera toxin B subunit binding does not correlate with GM1 expression: a study using mouse embryonic neural precursor cells. Glycobiology. 16 19G–22G. [DOI] [PubMed] [Google Scholar]
- 100.Usuki S., M. Pajaniappan, S. A. Thompson, and R. K. Yu. 2007. Chemical validation of molecular mimicry: interaction of cholera toxin with Campylobacter lipooligosaccharides. Glycoconj. J. 24 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanagisawa M., T. Ariga, and R. K. Yu. 2006. Fucosyl-GM1 expression and amyloid-β protein accumulation in PC12 cells. J. Neurosci. Res. 84 1343–1349. [DOI] [PubMed] [Google Scholar]
- 102.Masserini M., E. Freire, P. Palestini, E. Calappi, and G. Tettamanti. 1992. Fuc-GM1 ganglioside mimics the receptor function of GM1 for cholera toxin. Biochemistry. 31 2422–2426. [DOI] [PubMed] [Google Scholar]
- 103.Margolis R. U., M. Mazzulla, L. A. Greene, and R. K. Margolis. 1984. Fucosyl gangliosides of PC12 pheochromocytoma cells. FEBS Lett. 172 339–342. [DOI] [PubMed] [Google Scholar]
- 104.Ariga T., K. Kobayashi, Y. Kuroda, R. K. Yu, M. Suzuki, H. Kitagawa, F. Inagaki, and T. Miyatake. 1987. Characterization of tumor-associated fucogangliosides from PC 12 pheochromocytoma cells. J. Biol. Chem. 262 14146–14153. [PubMed] [Google Scholar]
- 105.Yamazaki Y., Y. Horibata, Y. Nagatsuka, Y. Hirabayashi, and T. Hashikawa. 2007. Fucoganglioside α-fucosyl(α-galactosyl)-GM1: a novel member of lipid membrane microdomain components involved in PC12 cell neuritogenesis. Biochem. J. 407 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ariga T., and R. K. Yu. 1999. GM1 inhibits amyloid β-protein-induced cytokine release. Neurochem. Res. 24 219–226. [DOI] [PubMed] [Google Scholar]
- 107.Liu Z., Y. Ruan, W. Yue, Z. Zhu, T. Hartmann, K. Beyreuther, and D. Zhang. 2006. GM1 up-regulates Ubiquilin 1 expression in human neuroblastoma cells and rat cortical neurons. Neurosci. Lett. 407 59–63. [DOI] [PubMed] [Google Scholar]
- 108.Hakomori S. 2004. Glycosynapses: microdomains controlling carbohydrate-dependent cell adhesion and signaling. An. Acad. Bras. Cienc. 76 553–572. [DOI] [PubMed] [Google Scholar]
- 109.Simons K., and W. L. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33 269–295. [DOI] [PubMed] [Google Scholar]
- 110.Simons K., and G. van Meer. 1988. Lipid sorting in epithelial cells. Biochemistry. 27 6197–6202. [DOI] [PubMed] [Google Scholar]
- 111.Prinetti A., K. Iwabuchi, and S. Hakomori. 1999. Glycosphingolipid-enriched signaling domain in mouse neuroblastoma Neuro2a cells. Mechanism of ganglioside-dependent neuritogenesis. J. Biol. Chem. 274 20916–20924. [DOI] [PubMed] [Google Scholar]
- 112.Jacobson K., and C. Dietrich. 1999. Looking at lipid rafts? Trends Cell Biol. 9 87–91. [DOI] [PubMed] [Google Scholar]
- 113.Simons K., and R. Ehehalt. 2002. Cholesterol, lipid rafts, and disease. J. Clin. Invest. 110 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hakomori S. I. 2000. Cell adhesion/recognition and signal transduction through glycosphingolipid microdomain. Glycoconj. J. 17 143–151. [DOI] [PubMed] [Google Scholar]
- 115.Hakomori S., and K. Handa. 2003. Interaction of glycosphingolipids with signal transducers and membrane proteins in glycosphingolipid-enriched microdomains. Methods Enzymol. 363 191–207. [DOI] [PubMed] [Google Scholar]
- 116.Rouvinski A., I. Gahali-Sass, I. Stav, E. Metzer, H. Atlan, and A. Taraboulos. 2003. Both raft- and non-raft proteins associate with CHAPS-insoluble complexes: some APP in large complexes. Biochem. Biophys. Res. Commun. 308 750–758. [DOI] [PubMed] [Google Scholar]
- 117.Schuck S., M. Honsho, K. Ekroos, A. Shevchenko, and K. Simons. 2003. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA. 100 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radeva G., and F. J. Sharom. 2004. Isolation and characterization of lipid rafts with different properties from RBL-2H3 (rat basophilic leukemia) cells. Biochem. J. 380 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Almeida R. F., L. M. Loura, A. Fedorov, and M. Prieto. 2005. Lipid rafts have different sizes depending on membrane composition: a time-resolved fluorescence resonance energy transfer study. J. Mol. Biol. 346 1109–1120. [DOI] [PubMed] [Google Scholar]
- 120.Vyas K. A., H. V. Patel, A. A. Vyas, and R. L. Schnaar. 2001. Segregation of gangliosides GM1 and GD3 on cell membranes, isolated membrane rafts, and defined supported lipid monolayers. Biol. Chem. 382 241–250. [DOI] [PubMed] [Google Scholar]
- 121.Pike L. J. 2004. Lipid rafts: heterogeneity on the high seas. Biochem. J. 378 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gomez-Mouton C., J. L. Abad, E. Mira, R. A. Lacalle, E. Gallardo, S. Jimenez-Baranda, I. Illa, A. Bernad, S. Manes, and A. C. Martinez. 2001. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA. 98 9642–9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ariga T. 2004. The role of caveolae in neurodegenerative diseases: glycosphingolipid homeostasis. Recent. Res. Devel. Neurochem. 7 97–108. [Google Scholar]
- 124.Molander-Melin M., K. Blennow, N. Bogdanovic, B. Dellheden, J. E. Mansson, and P. Fredman. 2005. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J. Neurochem. 92 171–182. [DOI] [PubMed] [Google Scholar]
- 125.Kalka D., C. von Reitzenstein, J. Kopitz, and M. Cantz. 2001. The plasma membrane ganglioside sialidase cofractionates with markers of lipid rafts. Biochem. Biophys. Res. Commun. 283 989–993. [DOI] [PubMed] [Google Scholar]
- 126.Kilkus J., R. Goswami, F. D. Testai, and G. Dawson. 2003. Ceramide in rafts (detergent-insoluble fraction) mediates cell death in neurotumor cell lines. J. Neurosci. Res. 72 65–75. [DOI] [PubMed] [Google Scholar]
- 127.Mitsutake S., M. Tani, N. Okino, K. Mori, S. Ichinose, A. Omori, H. Iida, T. Nakamura, and M. Ito. 2001. Purification, characterization, molecular cloning, and subcellular distribution of neutral ceramidase of rat kidney. J. Biol. Chem. 276 26249–26259. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y., K. Yamaguchi, T. Wada, K. Hata, X. Zhao, T. Fujimoto, and T. Miyagi. 2002. A close association of the ganglioside-specific sialidase Neu3 with caveolin in membrane microdomains. J. Biol. Chem. 277 26252–26259. [DOI] [PubMed] [Google Scholar]
- 129.Lee S. J., U. Liyanage, P. E. Bickel, W. Xia, P. T. Lansbury, Jr., and K. S. Kosik. 1998. A detergent-insoluble membrane compartment contains Aβ in vivo. Nat. Med. 4 730–734. [DOI] [PubMed] [Google Scholar]
- 130.Parkin E. T., A. J. Turner, and N. M. Hooper. 1999. Amyloid precursor protein, although partially detergent-insoluble in mouse cerebral cortex, behaves as an atypical lipid raft protein. Biochem. J. 344 23–30. [PMC free article] [PubMed] [Google Scholar]
- 131.Hayashi H., T. Mizuno, M. Michikawa, C. Haass, and K. Yanagisawa. 2000. Amyloid precursor protein in unique cholesterol-rich microdomains different from caveolae-like domains. Biochim. Biophys. Acta. 1483 81–90. [DOI] [PubMed] [Google Scholar]
- 132.Ehehalt R., P. Keller, C. Haass, C. Thiele, and K. Simons. 2003. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 160 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hattori C., M. Asai, H. Onishi, N. Sasagawa, Y. Hashimoto, T. C. Saido, K. Maruyama, S. Mizutani, and S. Ishiura. 2006. BACE1 interacts with lipid raft proteins. J. Neurosci. Res. 84 912–917. [DOI] [PubMed] [Google Scholar]
- 134.Wahrle S., P. Das, A. C. Nyborg, C. McLendon, M. Shoji, T. Kawarabayashi, L. H. Younkin, S. G. Younkin, and T. E. Golde. 2002. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9 11–23. [DOI] [PubMed] [Google Scholar]
- 135.Marlow L., M. Cain, M. A. Pappolla, and K. Sambamurti. 2003. β-secretase processing of the Alzheimer's amyloid protein precursor (APP). J. Mol. Neurosci. 20 233–239. [DOI] [PubMed] [Google Scholar]
- 136.Watanabe N., W. Araki, D. H. Chui, T. Makifuchi, Y. Ihara, and T. Tabira. 2004. Glypican-1 as an Aβ binding HSPG in the human brain: its localization in DIG domains and possible roles in the pathogenesis of Alzheimer's disease. FASEB J. 18 1013–1015. [DOI] [PubMed] [Google Scholar]
- 137.Kim S. I., J. S. Yi, and Y. G. Ko. 2006. Amyloid β oligomerization is induced by brain lipid rafts. J. Cell. Biochem. 99 878–889. [DOI] [PubMed] [Google Scholar]
- 138.Vetrivel K. S., H. Cheng, S. H. Kim, Y. Chen, N. Y. Barnes, A. T. Parent, S. S. Sisodia, and G. Thinakaran. 2005. Spatial segregation of γ-secretase and substrates in distinct membrane domains. J. Biol. Chem. 280 25892–25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tikkanen R., A. Icking, P. Beicht, G. L. Waneck, and H. Volker. 2002. The receptor-bound N-terminal ectodomain of the amyloid precursor protein is associated with membrane rafts. Biol. Chem. 383 1855–1864. [DOI] [PubMed] [Google Scholar]
- 140.Morishima-Kawashima M., and Y. Ihara. 1998. The presence of amyloid β-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry. 37 15247–15253. [DOI] [PubMed] [Google Scholar]
- 141.Kawarabayashi T., M. Shoji, L. H. Younkin, L. Wen-Lang, D. W. Dickson, T. Murakami, E. Matsubara, K. Abe, K. H. Ashe, and S. G. Younkin. 2004. Dimeric amyloid β protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. 24 3801–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ikezu T., B. D. Trapp, K. S. Song, A. Schlegel, M. P. Lisanti, and T. Okamoto. 1998. Caveolae, plasma membrane microdomains for α-secretase-mediated processing of the amyloid precursor protein. J. Biol. Chem. 273 10485–10495. [DOI] [PubMed] [Google Scholar]
- 143.Riddell D. R., G. Christie, I. Hussain, and C. Dingwall. 2001. Compartmentalization of β-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr. Biol. 11 1288–1293. [DOI] [PubMed] [Google Scholar]
- 144.Vetrivel K. S., H. Cheng, W. Lin, T. Sakurai, T. Li, N. Nukina, P. C. Wong, H. Xu, and G. Thinakaran. 2004. Association of γ-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem. 279 44945–44954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cordy J. M., I. Hussain, C. Dingwall, N. M. Hooper, and A. J. Turner. 2003. Exclusively targeting β-secretase to lipid rafts by GPI-anchor addition up-regulates β-site processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 100 11735–11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kametaka S., M. Shibata, K. Moroe, S. Kanamori, Y. Ohsawa, S. Waguri, P. J. Sims, K. Emoto, M. Umeda, and Y. Uchiyama. 2003. Identification of phospholipid scramblase 1 as a novel interacting molecule with β-secretase (β-site amyloid precursor protein (APP) cleaving enzyme (BACE)). J. Biol. Chem. 278 15239–15245. [DOI] [PubMed] [Google Scholar]
- 147.Parkin E. T., I. Hussain, E. H. Karran, A. J. Turner, and N. M. Hooper. 1999. Characterization of detergent-insoluble complexes containing the familial Alzheimer's disease-associated presenilins. J. Neurochem. 72 1534–1543. [DOI] [PubMed] [Google Scholar]
- 148.Ledesma M. D., J. S. Da Silva, K. Crassaerts, A. Delacourte, B. De Strooper, and C. G. Dotti. 2000. Brain plasmin enhances APP α-cleavage and Aβ degradation and is reduced in Alzheimer's disease brains. EMBO Rep. 1 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ledesma M. D., J. S. Da Silva, A. Schevchenko, M. Wilm, and C. G. Dotti. 2003. Proteomic characterisation of neuronal sphingolipid-cholesterol microdomains: role in plasminogen activation. Brain Res. 987 107–116. [DOI] [PubMed] [Google Scholar]
- 150.De Felice F. G., M. N. Vieira, L. M. Saraiva, J. D. Figueroa-Villar, J. Garcia-Abreu, R. Liu, L. Chang, W. L. Klein, and S. T. Ferreira. 2004. Targeting the neurotoxic species in Alzheimer's disease: inhibitors of Aβ oligomerization. FASEB J. 18 1366–1372. [DOI] [PubMed] [Google Scholar]
- 151.Yu W., K. Zou, J. S. Gong, M. Ko, K. Yanagisawa, and M. Michikawa. 2005. Oligomerization of amyloid β-protein occurs during the isolation of lipid rafts. J. Neurosci. Res. 80 114–119. [DOI] [PubMed] [Google Scholar]
- 152.Walkley S. U., M. Zervas, and S. Wiseman. 2000. Gangliosides as modulators of dendritogenesis in normal and storage disease-affected pyramidal neurons. Cereb. Cortex. 10 1028–1037. [DOI] [PubMed] [Google Scholar]
- 153.Mizuno T., M. Nakata, H. Naiki, M. Michikawa, R. Wang, C. Haass, and K. Yanagisawa. 1999. Cholesterol-dependent generation of a seeding amyloid β-protein in cell culture. J. Biol. Chem. 274 15110–15114. [DOI] [PubMed] [Google Scholar]
- 154.Kakio A., S. I. Nishimoto, K. Yanagisawa, Y. Kozutsumi, and K. Matsuzaki. 2001. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J. Biol. Chem. 276 24985–24990. [DOI] [PubMed] [Google Scholar]
- 155.Kakio A., S. Nishimoto, Y. Kozutsumi, and K. Matsuzaki. 2003. Formation of a membrane-active form of amyloid β-protein in raft-like model membranes. Biochem. Biophys. Res. Commun. 303 514–518. [DOI] [PubMed] [Google Scholar]
- 156.Hayashi H., N. Kimura, H. Yamaguchi, K. Hasegawa, T. Yokoseki, M. Shibata, N. Yamamoto, M. Michikawa, Y. Yoshikawa, K. Terao, et al. 2004. A seed for Alzheimer amyloid in the brain. J. Neurosci. 24 4894–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamamoto N., T. Yokoseki, M. Shibata, H. Yamaguchi, and K. Yanagisawa. 2005. Suppression of Aβ deposition in brain by peripheral administration of Fab fragments of anti-seed antibody. Biochem. Biophys. Res. Commun. 335 45–47. [DOI] [PubMed] [Google Scholar]
- 158.Mazur-Kolecka B., A. Golabek, K. Nowicki, M. Flory, and J. Frackowiak. 2006. Amyloid-β impairs development of neuronal progenitor cells by oxidative mechanisms. Neurobiol. Aging. 27 1181–1192. [DOI] [PubMed] [Google Scholar]
- 159.Zou K., J. S. Gong, K. Yanagisawa, and M. Michikawa. 2002. A novel function of monomeric amyloid β-protein serving as an antioxidant molecule against metal-induced oxidative damage. J. Neurosci. 22 4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lopez-Toledano M. A., and M. L. Shelanski. 2004. Neurogenic effect of β-amyloid peptide in the development of neural stem cells. J. Neurosci. 24 5439–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Haughey N. J., A. Nath, S. L. Chan, A. C. Borchard, M. S. Rao, and M. P. Mattson. 2002. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J. Neurochem. 83 1509–1524. [DOI] [PubMed] [Google Scholar]
- 162.Calafiore M., G. Battaglia, A. Zappala, E. Trovato-Salinaro, F. Caraci, M. Caruso, C. Vancheri, M. A. Sortino, F. Nicoletti, and A. Copani. 2006. Progenitor cells from the adult mouse brain acquire a neuronal phenotype in response to β-amyloid. Neurobiol. Aging. 27 606–613. [DOI] [PubMed] [Google Scholar]
- 163.Sugaya K., A. Alvarez, A. Marutle, Y. D. Kwak, and E. Choumkina. 2006. Stem cell strategies for Alzheimer's disease therapy. Panminerva Med. 48 87–96. [PubMed] [Google Scholar]
- 164.Yanagisawa M., T. Taga, K. Nakamura, T. Ariga, and R. K. Yu. 2005. Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. J. Neurochem. 95 1311–1320. [DOI] [PubMed] [Google Scholar]
- 165.Svennerholm L. 1994. Gangliosides—a new therapeutic agent against stroke and Alzheimer's disease. Life Sci. 55 2125–2134. [DOI] [PubMed] [Google Scholar]
- 166.Svennerholm L., G. Brane, I. Karlsson, A. Lekman, I. Ramstrom, and C. Wikkelso. 2002. Alzheimer disease – effect of continuous intracerebroventricular treatment with GM1 ganglioside and a systematic activation programme. Dement. Geriatr. Cogn. Disord. 14 128–136. [DOI] [PubMed] [Google Scholar]
- 167.Augustinsson L. E., K. Blennow, C. Blomstrand, G. Brane, R. Ekman, P. Fredman, I. Karlsson, M. Kihlgren, W. Lehmann, A. Lekman, et al. 1997. Intracerebroventricular administration of GM1 ganglioside to presenile Alzheimer patients. Dement. Geriatr. Cogn. Disord. 8 26–33. [DOI] [PubMed] [Google Scholar]
- 168.Gottfries C. G. 1994. Therapy options in Alzheimer's disease. Br. J. Clin. Pract. 48 327–330. [PubMed] [Google Scholar]
- 169.Matsuoka Y., M. Saito, J. LaFrancois, M. Saito, K. Gaynor, V. Olm, L. Wang, E. Casey, Y. Lu, C. Shiratori, et al. 2003. Novel therapeutic approach for the treatment of Alzheimer's disease by peripheral administration of agents with an affinity to β-amyloid. J. Neurosci. 23 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saulino M. F., and C. L. Schengrund. 1994. Differential accumulation of gangliosides by the brains of MPTP-lesioned mice. J. Neurosci. Res. 37 384–391. [DOI] [PubMed] [Google Scholar]
- 171.Schneider J. S., A. Pope, K. Simpson, J. Taggart, M. G. Smith, and L. DiStefano. 1992. Recovery from experimental Parkinsonism in primates with GM1 ganglioside treatment. Science. 256 843–846. [DOI] [PubMed] [Google Scholar]
- 172.Kharlamov A., I. Zivkovic, A. Polo, D. M. Armstrong, E. Costa, and A. Guidotti. 1994. LIGA20, a lyso derivative of ganglioside GM1, given orally after cortical thrombosis reduces infarct size and associated cognition deficit. Proc. Natl. Acad. Sci. USA. 91 6303–6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Polo A., G. Kirschner, A. Guidotti, and E. Costa. 1994. Brain content of glycosphingolipids after oral administration of monosialogangliosides GM1 and LIGA20 to rats. Mol. Chem. Neuropathol. 21 41–53. [DOI] [PubMed] [Google Scholar]
- 174.Wu G., Z. H. Lu, J. Wang, Y. Wang, X. Xie, M. F. Meyenhofer, and R. W. Ledeen. 2005. Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: protection with LIGA 20, a membrane-permeant analog of GM1. J. Neurosci. 25 11014–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Seren M. S., A. Lazzaro, C. L. Yang, R. Canella, M. Bassan, R. Zanoni, and H. Manev. 1994. Orally administered glycolipid derivative LIGA20 reduces infarct volume and behavioral impairment after focal cerebral ischemia. J. Pharmacol. Exp. Ther. 268 460–465. [PubMed] [Google Scholar]
- 176.Fazzini E., R. Durso, H. Davoudi, G. K. Szabo, and M. L. Albert. 1990. GM1 gangliosides alter acute MPTP-induced behavioral and neurochemical toxicity in mice. J. Neurol. Sci. 99 59–68. [DOI] [PubMed] [Google Scholar]
- 177.Pope-Coleman A., J. P. Tinker, and J. S. Schneider. 2000. Effects of GM1 ganglioside treatment on pre- and postsynaptic dopaminergic markers in the striatum of parkinsonian monkeys. Synapse. 36 120–128. [DOI] [PubMed] [Google Scholar]
- 178.Rothblat D. S., and J. S. Scneider. 1999. Regional differences in striatal dopamine uptake and release associated with recovery from MPTP-induced parkinsonism: an in vivo electrochemical study. J. Neurochem. 72 724–733. [DOI] [PubMed] [Google Scholar]
- 179.Pope-Coleman A., and J. S. Schneider. 1998. Effects of chronic GM1 ganglioside treatment on cognitieve and motor deficits in a slowly progressing model of Parkinsonism in non-human primates. Restor. Neurol. Neurosci. 12 255–266. [PubMed] [Google Scholar]
- 180.Schneider J. S., D. P. Roeltgen, E. L. Mancall, J. Chapas-Crilly, D. S. Rothblat, and G. T. Tatarian. 1998. Parkinson's disease: improved function with GM1 ganglioside treatment in a randomized placebo-controlled study. Neurology. 50 1630–1636. [DOI] [PubMed] [Google Scholar]
- 181.Schneider J. S., K. A. Bradbury, Y. Anada, J. Inokuchi, and D. W. Anderson. 2006. The synthetic ceramide analog L-PDMP partially protects striatal dopamine levels but does not promote dopamine neuron survival in murine models of parkinsonism. Brain Res. 1099 199–205. [DOI] [PubMed] [Google Scholar]
- 182.Odaka M., N. Yuki, E. Nobile-Orazio, M. Carpo, and K. Hirata. 2000. Antibodies to GM1(NeuGc) in Guillain-Barrè syndrome after ganglioside therapy. J. Neurol. Sci. 175 96–106. [DOI] [PubMed] [Google Scholar]
- 183.Svennerholm L. 1964. The gangliosides. J. Lipid Res. 5 145–155. [PubMed] [Google Scholar]