Abstract
Curcumin is a natural phenolic component of yellow curry spice, which is used in some cultures for the treatment of diseases associated with oxidative stress and inflammation. Curcumin has been reported to be capable of preventing the death of neurons in animal models of neurodegenerative disorders, but its possible effects on developmental and adult neuroplasticity are unknown. In the present study, we investigated the effects of curcumin on mouse multi-potent neural progenitor cells (NPC) and adult hippocampal neurogenesis. Curcumin exerted biphasic effects on cultured NPC; low concentrations stimulated cell proliferation, whereas high concentrations were cytotoxic. Curcumin activated extracellular signal-regulated kinases (ERKs) and p38 kinases, cellular signal transduction pathways known to be involved in the regulation of neuronal plasticity and stress responses. Inhibitors of ERKs and p38 kinases effectively blocked the mitogenic effect of curcumin in NPC. Administration of curcumin to adult mice resulted in a significant increase in the number of newly generated cells in the dentate gyrus of hippocampus, indicating that curcumin enhances adult hippocampal neurogenesis. Our findings suggest that curcumin can stimulate developmental and adult hippocampal neurogenesis, and a biological activity that may enhance neural plasticity and repair.
Neural progenitor cells (NPC)2 are the source of the neurons and glial cells that form all brain regions during embryonic development (1). However, the adult brain retains populations of NPC in the hippocampus and subventricular region of the cerebral cortex that are capable of dividing, migrating, and differentiating into neurons (2). NPC are known to respond to several types of environmental stimuli including physical exercise (3, 4), dietary restriction (5–7), and injury (8–10) by increasing their proliferation and/or the survival of newly generated neurons. On the other hand, chronic uncontrollable stress can impair hippocampal neurogenesis (11, 12). A better understanding of the factors that affect neurogenesis, and the underlying cellular and molecular mechanisms may lead to the development of restorative therapies for CNS injury and neurodegenerative disorders such as Alzheimer diseases (AD).
Curcumin (diferuloylmethane) is a naturally occurring phenolic yellow chemical isolated from the rhizomes of the plant Curcuma longa Linn (turmeric), and is a major component of the spice turmeric. Turmeric has traditionally been used in India for the treatment of diseases associated with injury and inflammation (13). Because of its ability to scavenge free radicals and inhibit inflammation (14–16), curcumin has been investigated for cancer chemoprevention and tumor growth suppression. Recent findings suggest the possibility that curcumin can reduce oxidative damage and cognitive deficits associated with aging (17–19). Studies of animal models have suggested that curcumin may be beneficial in neurodegenerative conditions such as AD (20–22) and focal cerebral ischemia (23). In addition, the curcumin treatment can protect hippocampal neurons against excitotoxic and traumatic injury (24, 25).
In addition to the direct free radical scavenging properties of high (micromolar) concentrations of curcumin, lower concentrations can activate or inhibit one or more signal transduction pathways in cells. In tumor cells, curcumin can inhibit growth factor-mediated signaling pathways including those coupled to extracellular-regulated kinases (26, 27) and protein kinase C (28). On the other hand, curcumin can activate the Nrf2-ARE and p38 MAP kinase pathways in tumor cells resulting in the induction of the expression of phase 2 enzymes such as heme oxygenase-1 (29). Studies of the effects of curcumin on neurons suggest that it can induce the expression of cytoprotective proteins such as heme oxygenase-1 (30). Emerging evidence suggests that some phytochemicals exert their health-promoting effects by activating one or more adaptive cellular stress response pathways (31). Because several of those pathways, including those involving MAP kinase, are known to regulate neurogenesis (32), we explored the effects of curcumin on NPC in culture and in vivo. Our findings demonstrate that curcumin can enhance neurogenesis via an ERK- and p38 MAP kinase-mediated mechanism.
EXPERIMENTAL PROCEDURES
Cell Culture Methods—C17.2 cells are multipotent NPC, which were isolated from the neonatal mouse cerebellum and immortalized (33). C17.2 NPC have been widely used as cell transplantation candidates for the treatment of several neurodegenerative disorders because they are able to integrate in the host adult brain, and differentiate into multiple stable cell phenotypes after grafting (34, 35). The C17.2 NPC line (a generous gift from C. Cepko) was maintained in plastic culture flasks in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 5% horse serum, and 2 mm l-glutamine in a humidified atmosphere of 5% CO2, 95% air at 37 °C. Cancer cell lines including embryonic carcinoma cells (P19), mouse hepatoma cells (H1c1c7), and human hepatoma cells (HepG2) were grown in DMEM containing 10% fetal bovine serum and 2 mm l-glutamine in a humidified atmosphere of 5% CO2, 95% air at 37 °C. Glioma cells (C6) were grown in the same conditions as the C17.2 NPC. PD98059, SB203580, and SP600125 were purchased from Sigma. Treatments were administered by direct dilution into the culture medium, and an equivalent volume of vehicle was added to control cultures. To establish cultures of primary embryonic cortical neural stem cells, pregnant C57BL/6 mice were sacrificed on gestational day 13 by cervical dislocation, and embryos were collected. The brains removed from the embryos were collected into ice-cold Ca2+- and Mg2+-free Hank's Balanced Saline Solution (HBSS; WelGENE Inc., Daegu, Korea) containing 0.1 mg/ml gentamycin. Meninges were removed from the brain, and the cortical neuroepithelium was dissected and collected in cold HBSS. After centrifugation, brain tissue was resuspended in 2 mg/ml trypsin/EDTA solution. Trypsinization was performed under gentle agitation for 12–15 min at room temperature, and the reaction was stopped by adding of 1.5 mg/ml trypsin inhibitor in HBSS. The cells were dissociated by gentle trituration with a fire-polished Pasteur pipette to yield suspensions of single cells or small cell clusters. The dissociated cells were diluted in culture medium (DMEM/F12 (WelGENE Inc.) containing B27 supplements and basic fibroblast growth factor (40 ng/ml; Invitrogen) and plated into 96-well plates or plastic culture dishes at the desired cell densities.
Cell Proliferation Assay—Cell proliferation was assessed by colorimetric assay using MTT assay, which is based on the reduction of MTT by the mitochondrial dehydrogenase of intact cells into an insoluble purple formazan product. Cells were seeded at a density of 1 × 104 cells in 96-well plates. After 24 h, the cells were treated with different concentrations of curcumin (0.1, 0.5, 1, 10, 20, and 50 μm). After the treatment, medium containing curcumin was removed, cells were washed twice with PBS, and 200 μl of 0.5 mg/ml MTT in PBS was added to each well. The plate was incubated at 37 °C for 4 h. Then, the cells were disrupted in solubilization solution (dimethyl sulfoxide (DMSO) and ethanol (EtOH), 1:1 ratio). The formazan dye produced by viable cells was quantified in an ELISA microplate reader at an absorbance of 560 nm.
Protein Analysis and Western Blotting—The protein concentration was determined by the Lowry method (Sigma) using bovine serum albumin as a standard. Homogenized and lysed samples were boiled for 5 min in a gel-loading buffer (125 mm Tris-HCl, 4% sodium dodecyl sulfate (SDS), 10% 2-mercaptoethanol, and 0.2% bromphenol blue, pH 6.8) at a sample/buffer ratio of 1:1. Equal amounts of protein were separated by SDS-PAGE using 6–17% gels. The proteins were transferred from the gel onto an Immobilon-PSQ transfer membrane (Millipore Corp, Bedford, MA). The membrane was immediately placed in a blocking solution (5% nonfat dry milk in TBS-Tween (TBS-T) buffer containing 10 mm Tris, 100 mm NaCl, and 0.1% Tween 20, pH 7.5) for 1 h at room temperature. The membrane was washed in TBS-T buffer for 30 min and then incubated with primary antibody at room temperature for 4 h. After two 30-min washes in TBS-T buffer, the membrane was incubated with a secondary antibody for 2 h at room temperature. After washing for 40 min in TBS-T buffer, antibody labeling was detected by enhanced chemiluminescence (ECL) and exposed to radiographic film. Prestained blue protein markers were used for molecular weight determination. The primary antibodies included: mouse monoclonal antibodies against phospho-ERK, phospho-p38, phospho-JNK (Santa Cruz Biotechnology, Santa Cruz, CA; 1:500 dilution); rabbit polyclonal antibodies against ERK, p38, JNK (Cell Signaling Technology, Danvers, MA; 1:500 dilution); a mouse monoclonal antibody against β-actin (Cell Signaling Technology, 1:2500 dilution).
BrdU Immunocytochemistry—For labeling cells, BrdU was added to the culture medium to a final concentration of 20 μm at 37 °C for 2 h and washed with culture medium without BrdU. Cells were then treated with either vehicle or curcumin. Twenty-four hours later, cultured cells were then fixed in 4% paraformaldehyde in PBS, pH 7.3 and washed with PBS. For immunostaining, fixed cells were postfixed in 70% ethanol (in 50 mm glycine buffer, pH 2.0) at -20 °C for 20 min. Then 0.6% H2O2 in TBS was added to block endogenous peroxidases. DNA was denatured by sequentially exposing cells to heat (65 °C), acid (2 m HCl), and base (0.1 m borate buffer). The primary BrdU antibody (rat monoclonal; Accurate Chemicals, Westbury, NY; 1:400) was added, and the plate was incubated overnight at 4 °C. The cells were then washed with TBS and incubated for 2 h in the presence of biotinylated secondary antibody. The cells, then, were reacted with ABC solution (Vector Laboratories, Inc. Burlingame, CA) for 1 h at room temperature. Color development was assessed using a DAB kit according to the manufacturer's instructions. Cells were visualized, and images acquired using a Zeiss 510 CSLM microscope.
Mice and Curcumin Administration—Adult (7-week-old) male C57BL/6 mice obtained from Daehan Biolink (Chungbuk, Korea) were maintained under temperature- and light-controlled conditions (20–23 °C, 12-h light/12-h dark cycle) and provided food and water ad libitum. Mice were divided into two groups for vehicle and curcumin treatment and acclimatized for 1 week prior to treatment. Curcumin was administered intraperitoneally at a dose of 500 nmol/kg body weight, once daily for 4 days. Saline was used as vehicle treatment. For evaluations of neurogenesis, mice from each group were given an intraperitoneal injection of BrdU (50 mg/kg body weight) twice daily for 3 days. Half of the mice in each treatment group were euthanized one day after the last BrdU injection, and half were euthanized 4 weeks after the last BrdU injection.
Tissue Preparation—At day 1 or 4 weeks after curcumin injection, mice were anesthetized, and then perfused intracardially with 4% paraformaldehyde (PFA) in PBS (pH 7.4). Following fixative perfusion, brains were removed from the cranium, placed in the same fixation solution at 4 °C overnight, and transferred to a 30% sucrose solution. The cryoprotected brains were sectioned serially at 40 μm in the coronal plane using a freezing microtome (MICROM), collected in Dulbecco's phosphate-buffered saline (DPBS) solution containing 0.1% sodium azide, and stored at 4 °C. Every section that contained the hippocampal formation was saved.
Quantification of Newly Generated Neural Cells—The protocol for immunostaining of brain sections with BrdU antibody was similar to that described previously (12). Briefly, free-floating sections were treated with 0.6% H2O2 in Tris-buffered saline (TBS; pH 7.5) to block endogenous peroxidases, and DNA was denatured by exposing sections sequentially to heat, acid, and base. The sections were incubated in TBS, 0.1% Triton X-100, 3% goat serum (TBS-TS) for 30 min, and incubated with primary anti-BrdU antibody (rat monoclonal; Accurate Chemicals, 1:400) in TBS-TS at 4 °C overnight. Sections were further processed using a biotinylated secondary goat anti-rat IgG antibody (Vector Laboratories, 1:200), avidin-peroxidase complex, and diaminobenzidine. Stained sections were mounted onto slides and counterstained with cresyl violet to measure granule cell layer area.
Double Label Immunostaining— For double labeling of BrdU and the neuronal marker NeuN, blocking with 3% normal goat serum (Vector Laboratories) was performed, and the sections were incubated with rat polyclonal anti-BrdU antibody (1:500 dilution; Accurate Chemical & Scientific Corporation) and mouse monoclonal anti-NeuN antibody (1:500 dilution; Chemicon, CA) at 4 °C overnight. For double labeling with astrocyte marker, rabbit polyclonal antibody against GFAP (Sigma, 1:500 dilution) was used. Brain sections were then washed with TBS and incubated for 2 h in the presence of anti-mouse IgG labeled with Alexia Fluor-488 and anti-rat IgG labeled with Alexia Fluor-568. Confocal fluorescence images were acquired using a Zeiss 510 CSLM microscope.
Statistical Analysis—The statistical significance of the differences between control and curcumin-treated cultured cells and mice was determined by analysis of variance (ANOVA) with Fisher protected least significant difference (PLSD). Values of p < 0.05 were considered statistically significant. Analyses were performed using Statview software®.
RESULTS
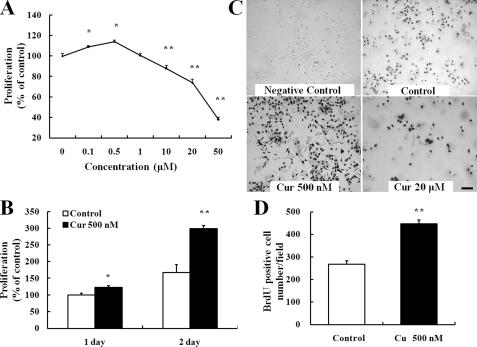
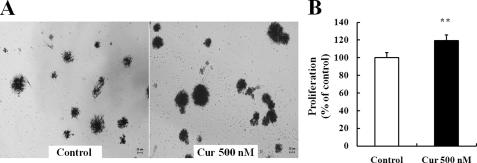
Curcumin Increases the Proliferation of C17.2 NPC—To examine the effect of curcumin on the proliferation of NPCs, C17.2 cells growing in 96-well or 6-well plates were maintained in medium lacking or containing increasing concentrations of curcumin (Fig. 1A), and the cell proliferation rate was quantified at different time points (Fig. 1B). After 24 h of curcumin treatment, lower doses (0.1, 0.5 μm) of curcumin increased NPC proliferation, whereas high doses (≥10 μm) caused a decrease in NPC proliferation (Fig. 1A). Among the concentrations tested, 500 nm curcumin was the most effective in stimulating NPC proliferation. The proliferative effects of low concentrations of curcumin, evaluated during a 2-day treatment period, revealed that the proliferation-promoting action of curcumin was progressively increased during the 2-day time period (Fig. 1B). BrdU immunocytochemistry was performed to confirm the proliferative effects of curcumin on NPCs (Fig. 1C). Quantification of nuclei immunostained with BrdU showed a significant increase of NPC proliferation in cultures treated with 500 nm curcumin (Fig. 1D). These findings indicate that low concentration curcumin induce the proliferation of NPCs, while high doses of curcumin are cytotoxic.
FIGURE 1.
Low concentrations of curcumin stimulate the proliferation of neural progenitor cells. A, C17.2 NPCs were seeded into 96-well culture plates and treated with the indicated concentrations of curcumin for 24 h. B, most effective dose curcumin (500 nm) was re-evaluated during a 2-day treatment period. Proliferation of NPCs was evaluated using the MTT assay method. Each value is the mean and S.E. (n = 8 cultures). C, images of BrdU immunoreactivity in cells treated with 20 μm BrdU (or vehicle control) for 2 h and then exposed to curcumin for 24 h. Scale bar, 100 μm. D, BrdU immunostained cells were counted in a blinded manner; values are the mean ± S.E. (n = 4 cultures). *, p < 0.05; **, p < 0.01 compared with corresponding value for control. Cur, curcumin.
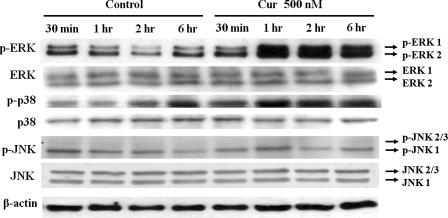
MAPK Signaling Mediates the Proliferative Effect of Curcumin on NPCs—Immunoblot analysis was performed to determine whether one or more MAP kinases are activated in response to curcumin in NPCs. Curcumin (500 nm) significantly increased the levels of phosphorylated ERK within 30 min, with a peak increase within 1 h, and a sustained elevation through 6 h (Fig. 2). Levels of phosphorylated p38 kinases were also elevated in NPC treated with 500 nm curcumin. However, phosphorylated JNK proteins were not significantly affected by curcumin treatment (Fig. 2). Interestingly, the ERK and p38 activation induced by curcumin were transient with activity levels returning to baseline within 24 h (supplemental Fig. S1).
FIGURE 2.
Evidence that curcumin induces the activation of ERK and p38 MAP kinases in neural progenitor cells. Whole cell extracts (35 μg/lane) from C17.2 NPC treated with the indicated concentrations of curcumin for the indicated time periods were subjected to immunoblot analysis using antibodies against phospho-ERK44/42, phospho-JNK54/46, or phospho-p38. Levels of β-actin were also determined as a control for possible protein loading variability. Blots are representative of four separate experiments.
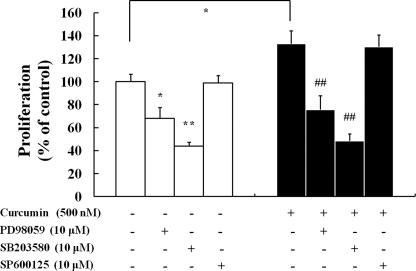
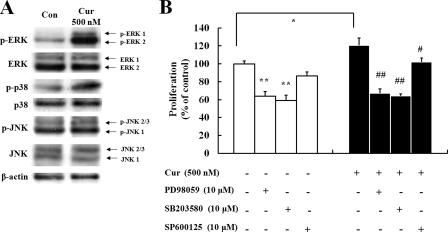
MAPK Inhibitors Effectively Block Curcumin-induced Proliferation in NPCs—To determine whether the activation of MAP kinases is required for proliferation of NPCs, NPCs were treated with the MEK/ERK inhibitor PD98059, the p38 inhibitor SB203580 or the JNK inhibitor SP600125 prior to curcumin treatment. Treatment with PD98059 and SB203580 completely blocked curcumin-induced proliferation of NPCs (Fig. 3), suggesting a requirement for ERKs and p38 MAP kinase in the action of curcumin. In contrast, the JNK inhibitor SP600125 had no significant effect on curcumin-induced cell proliferation. The inhibitors of ERK and p38 kinases were also effective in decreasing the proliferation of NPCs in the absence of curcumin, indicating that ERK and p38 kinases are also involved in the basal proliferation of NPCs. These findings indicate that ERK and p38 kinase activation is involved in the curcumin-mediated enhancement of proliferation in NPCs.
FIGURE 3.
ERK and p38 MAP kinase inhibitors block curcumin-induced proliferation of neural progenitor cells. C17.2 NPCs treated with the indicated kinase inhibitors for 12 h were then treated with 500 nm curcumin for 24 h, and MTT assays were performed. Inhibitors used were: SB203580 (p38 MAP kinase inhibitor), 10 μm; PD98059 (MEK/ERK inhibitor), 10 μm; and SP600125 (JNK inhibitor), 10 μm. Values are the mean and S.E. (n = 8 cultures). *, p < 0.05; **, p < 0.01 compared with corresponding value for control. ##, p < 0.01 compared with the value for cultures treated with curcumin alone.
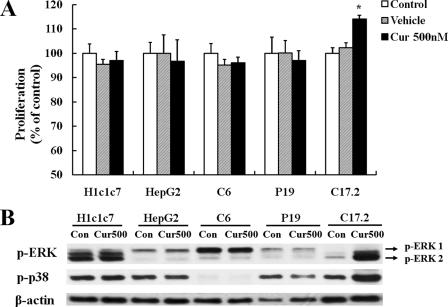
Cell Type-specific Proliferative Responses to Curcumin—Because previous studies of various cancer cell lines have suggested that curcumin can inhibit cell proliferation, we evaluated the effects of 500 nm curcumin on the proliferation of several cancer cell lines including glioma cells (C6), embryonic carcinoma cells (P19), mouse hepatoma cells (H1c1c7), and human hepatoma cells (HepG2). Cells were seeded into 96-well culture plates containing medium in the absence or presence of curcumin and cultured for 24 h. The results of MTT assays showed that there was no proliferative effect of 500 nm curcumin on any of the tested cancer cell lines, indicating the mitogenic actions of curcumin were NPC-specific (Fig. 4A). Moreover, immunoblot analysis indicated that curcumin did not activate MAPK signaling in the cancer cell lines (Fig. 4B).
FIGURE 4.
The proliferative action of curcumin is selective for neural progenitor cells. A, NPCs and the indicated lines of tumor cells (HepG2, human hepatoma; H1c1c7, mouse hepatoma; P19, embryonal carcinoma cells; C6, glioma cells) were seeded into 96-well culture plates and cultured for 24 h. The cells were then treated with 500 nm curcumin for 24 h, and cell proliferation was quantified using the MTT assay. Values are the mean and S.E. (n = 6 cultures). B, whole cell extracts (35 μg/lane) from untreated and curcumin-treated cells for 2 h were subjected to immunoblot analysis with antibodies against phospho-ERK44/42 and phospho-p38. Levels of β-actin were determined to evaluate protein loading. One representative blot is shown from three experiments that yielded similar results. *, p < 0.05 compared with corresponding value for control.
Curcumin Stimulates the Proliferation of Embryonic Cortical Neural Stem Cells via the MAP Kinase Pathways—The proliferative effect of curcumin was investigated on primary neural stem cells (NSCs) established from embryonic day 13 mouse cerebral neuroepithelium. When NSCs were treated with either vehicle or 500 nm curcumin for 24 h, increased neurosphere formation was observed after exposure to MTT solution (Fig. 5A). The proliferative effect of curcumin on NSCs was confirmed using the MTT assay (Fig. 5B). Because the ERK and p38 MAP kinase pathways mediated the proliferation-inducing effects of curcumin in C17.2 NPCs, we determined whether the same pathways mediated curcumin-induced proliferation of embryonic cortical NSC. Immunoblot analysis showed that 500 nm curcumin strongly activated ERK and p38 kinases, whereas JNK was not affected by curcumin treatment (Fig. 6A). The MEK/ERK inhibitor PD98059 and the p38 inhibitor SB203580 each effectively blocked the curcumin-induced proliferation of embryonic mouse NSCs as well as basal proliferation (Fig. 6B). Interestingly, unlike C17.2 NPC, the JNK inhibitor SP600125 had a small effect on the basal proliferation of NSC proliferation, and significantly blocked the curcumin-induced proliferation of embryonic mouse NSC (Fig. 6B). These data confirm that ERK and p38 kinases are more essential for cell proliferation in primary NSCs and are closely involved in the curcumin-mediated proliferation.
FIGURE 5.
Curcumin stimulates the proliferation of embryonic mouse cortical neural stem cells. Dissociated embryonic cortical cells were diluted in culture medium and plated into 96-well plates or 35-mm culture dishes and cultured for 2 days. The cells were treated with 500 nm curcumin for 24 h. A, neurospheres were grown in either vehicle- or curcumin-containing medium for 24 h. Images of expanded neurospheres were taken after exposure to MTT solution for 4 h. B, proliferation of NSCs was quantified by MTT analysis. Each value is the mean and S.E. (n = 8). MTT-positive cells were counted, and the values are the mean ± S.E. (n = 8). **, p < 0.01 compared with corresponding value for control cultures. Scale bar, 20 μm. Cur, curcumin.
FIGURE 6.
Activation of ERK and p38 MAP kinases mediates the proliferative effects of curcumin on embryonic cortical neural stem cells. A, whole cell extracts from cortical NSC cultures that had been treated for 2 h with 500 nm curcumin were subjected to immunoblotting with antibodies against phospho-ERK44/42, phospho-JNK54/46, or phospho-p38. Levels of total ERK, total p38, total JNK, and β-actin were used to evaluate protein loading. The blot shown is representative of results obtained in three separate experiments. B, ERK and p38 MAP kinase inhibitors block curcumin-induced proliferation of embryonic mouse cortical NSCs. After pretreatment with the indicated MAP kinase inhibitors for 12 h, NSCs were incubated with either vehicle or 500 nm curcumin for 24 h. NSC proliferation was assessed by MTT assay. Values are the mean and S.E. (n = 8). *, p < 0.05; **, p < 0.01 compared with the corresponding value for control. #, p < 0.05; ##, p < 0.01 compared with the value for cultures treated with curcumin alone.
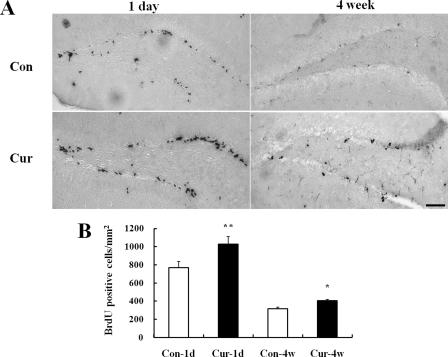
Curcumin Increases the Numbers of Newly Generated Cells in the Dentate Gyrus of the Hippocampus in Adult Mice—To elucidate the possible effects of curcumin on adult NSCs of the hippocampus, we employed 8-week-old C57BL/6 mice. Either curcumin or vehicle was administered intraperitoneally at a dose of 500 nmol/kg body weight, once daily for 4 days. Based on previously published data, we estimated that systemic administration of 500 nmol/kg body would result in a peak concentration of curcumin in the blood of 5–6 μm (22). Taking into consideration penetration of the blood-brain barrier, we would expect that a 500 nmol/kg systemic dose of curcumin would result in roughly a 1∼2 μm concentration in the brain. To label the newly generated cells, 6 doses of BrdU were given intraperitoneally for 3 days beginning on the second day of curcumin administration. Mice were sacrificed at either day 1 or 4 weeks after the last BrdU injection. The dividing cells labeled with BrdU were visualized by BrdU immunohistochemistry. At the day 1 time point, the numbers of BrdU-positive cells in the dentate gyrus were significantly greater in curcumin-treated mice compared with vehicle-treated control mice (Fig. 7, A and B). At the 4-week time point, there were slightly more BrdU-positive cells in the dentate gyrus of mice in the curcumin group compared with the control group (Fig. 7, A and B). At the 4-week time point, the BrdU-labeled cells were distributed throughout the entire granule cell layer, and their nuclei were large and round, characteristic of mature granule neurons.
FIGURE 7.
Curcumin administration increases the number of BrdU-positive cells in the hippocampus of adult mice. Adult mice that were treated with either vehicle (control) or curcumin were administered BrdU and then euthanized at either day 1 or 4 weeks later, and brain tissue sections were processed for BrdU immunostaining (see “Experimental Procedures”). A, photomicrographs of representative coronal brain sections showing BrdU-positive cells in the dentate gyrus of the hippocampus. Note that the numbers of BrdU-labeled cells appear greater in sections of curcumin-treated mice compared with vehicle-treated control mice. Scale bar, 100 μm. B, quantitative analysis of the number of BrdU-positive cells in the dentate gyrus of control and curcumin-treated mice at day 1 or 4 weeks after BrdU administration. Values are the mean and S.E. (n = 6 mice). *, p < 0.05; **, p < 0.01, compared with the corresponding value for group control. Cont, vehicle control; Cur, curcumin; 1D, day 1; 4W, 4 weeks.
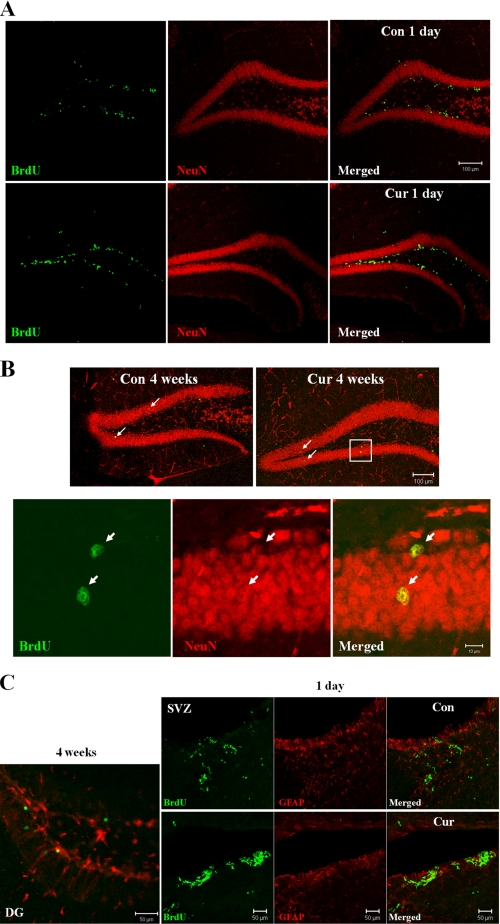
Curcumin Enhances Adult Hippocampal Neurogenesis—To determine the phenotypes of the newly generated cells, we performed double label fluorescence immunohistochemistry on the hippocampi using antibodies against the mature neuron-specific protein (NeuN) in combination with the BrdU antibody. At day 1 after BrdU administration, the vast majority of BrdU-positive cells was observed in the subgranular zone of dentate gyrus and was not co-labeled with the NeuN antibodies (Fig. 8A). At 4 weeks after BrdU administration, BrdU-positive cells were scattered throughout the dentate gyrus. Essentially all BrdU-positive cells that were located within the granule cell layer were NeuN-positive, suggesting that most of the cells that incorporated BrdU in the granule cell layer differentiated into mature neurons (Fig. 8B). To evaluate gliogenesis, double label immunostaining with BrdU and GFAP (astrocyte marker) antibodies was performed. We found that the majority of newly generated cells within dentate gyrus at 4 weeks after BrdU injection were not co-labeled with GFAP (Fig. 8C, left). These results indicate that the administration of curcumin promotes hippocampal neurogenesis in adult mice. Interestingly, we found that more BrdU-positive cells were detected in the subventricular region of curcumin-treated mice at day 1 after the last BrdU injection, suggesting that curcumin stimulates the proliferation of neural stem cells not only in dentate gyrus but also in the subventricular region of the cerebral cortex (Fig. 8C, right).
FIGURE 8.
Curcumin treatment enhances neurogenesis in the hippocampus of adult mice. A and B, to determine the phenotypes of the newly generated cells, brain sections from control and curcumin-treated mice were double labeled with fluorescent probes using antibodies against the mature neuron-specific protein (NeuN) and BrdU antibody. BrdU-positive cells (green, proliferation marker); NeuN-positive cells (red, mature neuron maker). Note the greater numbers of BrdU-labeled cells in the dentate gyrus of curcumin-treated mice one day after BrdU injection (A). Double-labeled cells (newly generated neurons: arrows) were also greater in number in sections from curcumin-treated mice compared with control mice. C, double label immunostaining with BrdU and GFAP (astrocyte marker) antibodies was performed. The majority of newly generated cells within dentate gyrus at 4 weeks after BrdU injection were not co-labeled with GFAP (left). Note that more BrdU-positive cells were seen in the subventricular zone of curcumin-treated mice at day 1 after the last BrdU injection (right). Scale bar, 100 μm (A and B top); scale bar, 10 μm (B bottom); scale bar, 50 μm (C). Cont, control; Cur, curcumin; DG, dentate gyrus; SVZ, subventricular zone.
DISCUSSION
Previous studies have documented antioxidant and anti-inflammatory effects of micromolar concentrations of curcumin in cultured tumor cell lines as well as normal non-neuronal cells (17–19, 36, 37) In addition, treatment of cell cultures and adult rodents with curcumin can protect neurons from being damaged and killed in models relevant to the pathogenesis of AD, Parkinson disease, and stroke (24, 38–40). The present findings demonstrate a novel biological action of curcumin, namely, enhancement of neurogenesis. Our data show that curcumin enhance the proliferation of C17.2 NPC and embryonic cortical NSC in culture, and NSC in the hippocampus of adult mice. Increasing evidence suggests that adult neurogenesis may play important roles in processes such as learning and memory. Hippocampal neurogenesis is increased in response to environmental conditions that also improve learning and memory environmental enrichment (12), exercise (4), and dietary energy restriction (7). In addition, pharmacological suppression of hippocampal neurogenesis is associated with an impairment of hippocampus-dependent memory in rodents (41).
The enhancement of hippocampal neurogenesis by curcumin documented in our study is similar to the positive effects of exercise and environmental enrichment, suggesting the possibility that curcumin might also enhance hippocampal function. Curcumin might also be beneficial in conditions where neurogenesis is compromised, including aging (42), AD (43), and diabetes (44). Indeed, curcumin ameliorated learning and memory deficits in a rat model of AD (45).
A concentration of 500 nm curcumin, which was the most effective for inducing the proliferation of C17.2 NPCs, also stimulated the proliferation of embryonic cortical NSCs. At concentrations above 10 μm curcumin inhibited NPC growth and was cytotoxic, effects similar to the cytotoxic effects of curcumin in various types of cancer cells (36, 37, 46–48). Our data suggest that the stimulatory effect of low doses of curcumin on neural stem cells is mediated by ERK and p38 MAP kinases. Curcumin increased the phosphorylation of ERKs and p38 MAP kinases indicating stimulation of the activity of these enzymes. The ability of curcumin to induce proliferation of neural stem cells was abolished by inhibitors of MEK/ERK and p38 MAP kinases, demonstrating a requirement for these kinases in the biological effect of curcumin on the stem cells. Previous studies have shown that MAP kinases mediate the proliferative effects of growth factors on neural stem cells (49). For example, basic fibroblast growth factor (FGF2) stimulates the proliferation of rat neural stem cells by an ERK-mediated mechanism (50), and FGF2 maintains mouse cortical NPC in a proliferative state by a mechanism involving ERK-mediated up-regulation of gap junctions (51). On the other hand, previous studies have not clearly established the biological functions of p38 MAP kinase in neural stem cells. Thus, p38 MAP kinase mediates apoptosis of C17.2 NPC triggered by nitric oxide (52), but promotes differentiation of mouse NSC (53) and mediates the proliferative effects of FGF2 and platelet-derived growth factor on oligodendrocyte progenitor cells (54). Interestingly, we found that there was no detectable increase in ERK or p38 MAP kinase activities in cancer cell lines in response to 500 nm curcumin. It has been previously shown that 10 μm curcumin treatment activates p38 MAPK in HUH7 human hepatoma cells (29). This study indicated that a ROS-generating concentration of curcumin induces HO-1 through p38 activation. However, the much lower concentration of 500 nm curcumin is not likely to generate ROS and activate the ROS stress related p38 activation in non-neural cancer cells tested in the current study. In light of previous findings showing that curcumin inhibits the proliferation of various types of cancer cells (55), our findings suggest that the effects of curcumin on cell proliferation and MAP kinase signaling in neural stem cells are cell type-specific. The reason for this differential effect of curcumin on neural stem cells and non-neural cancer cells remains to be determined.
The molecular mechanism by which curcumin activates ERK and p38 MAP kinases is unknown. One possibility is that curcumin directly interacts with the kinases themselves, or kinases or phosphatases upstream of the MAP kinases. Although this has not been established, there is precedence for direct interactions of electrophilic compounds such as polyphenols with signal transduction molecules. Previous studies suggest that electrophilic curcumin can activate the Nrf2-antioxidant response element pathway by interacting with and dissociating the Keap-Nrf2 complex (56). Alternatively, the activation of ERK and p38 MAP kinases may represent an adaptive response of the cells to stress induced by curcumin. There is considerable evidence that high (micromolar) concentrations of curcumin can induce oxidative stress, and that this may mediate its ability to trigger apoptosis in cancer cells (57). Subtoxic doses of curcumin may therefore induce a mild adaptive stress response that involves the activation of ERK and p38 MAP kinases resulting in increased proliferation and survival of neural stem cells. The latter mechanism is consistent with the hormesis hypothesis for the beneficial actions phytochemicals on neurons (31).
It was recently reported that curcumin treatment can reverse impaired hippocampal neurogenesis in chronically stressed rats (58). However, in the latter study, high doses (10 and 20 mg/kg) of curcumin were administered chronically, and the effects of curcumin were focused on the prevention of impaired hippocampal neurogenesis induced by chronic stress. Similarly, most previous in vivo studies of curcumin were focused on the ability of high doses of curcumin (5–200 mg/kg) to protect neurons against various types of stress (25, 30, 59). Interestingly, we found that a 40-fold higher dose of curcumin fails to increase the numbers of BrdU-positive cells and were not toxic to neural stem cells in adult hippocampus (supplemental Fig. S2). These data suggest that the level of curcumin treatment in vivo is critical to stimulate the proliferation of neural stem cell in the hippocampus. In addition, we found that there is no evidence of neuronal degeneration caused by high-dose curcumin treatment in the hippocampus (supplemental Fig. S3).
In the present study, we found that a much lower dose of curcumin (less than 0.2 mg/kg) significantly increased the proliferation of neural stem cells in adult hippocampus. Therefore, relatively low doses of curcumin can stimulate hippocampal neuroplasticity, a finding with important implications for preventative and therapeutic approaches for a range of neurological disorders that involve impaired neurogenesis, including depression (60), diabetes (44), and AD (43).
Supplementary Material
Acknowledgments
We thank C. Cepko at Harvard University (Boston, MA) for kindly providing the C17.2 cell line.
This work was authored, in whole or in part, by National Institutes of Health staff. This work was also supported by Grant No. R01-2005-000-10661-0 from the Basic Research Program of the Korea Science & Engineering Foundation and the Brain Korea 21 Project. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
Footnotes
The abbreviations used are: NPC, neural progenitor cell; AD, Alzheimer disease; ERK, extracellular signal-regulated kinase; GFAP, glial fibrillary acidic protein; JNK, c-Jun N-terminal kinase; MAP, mitogen-activated protein; NSC, neural stem cell; BrdU, bromodeoxyuridine; PBS, phosphate-buffered saline; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DMEM, Dulbecco's modified Eagle's medium; CNS, central nervous system.
References
- 1.Alvarez-Buylla, A., Garcia-Verdugo, J. M., and Tramontin, A. D. (2001) Nat. Rev. Neurosci. 2 287-293 [DOI] [PubMed] [Google Scholar]
- 2.Gould, E. (2007) Nat. Rev. Neurosci. 8 481-488 [DOI] [PubMed] [Google Scholar]
- 3.van Praag, H., Christie, B. R., Sejnowski, T. J., and Gage, F. H. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 13427-13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Praag, H., Kempermann, G., and Gage, F. H. (1999) Nat. Neurosci. 2 266-270 [DOI] [PubMed] [Google Scholar]
- 5.Lee, J., Duan, W., Long, J. M., Ingram, D. K., and Mattson, M. P. (2000) J. Mol. Neurosci. 15 99-108 [DOI] [PubMed] [Google Scholar]
- 6.Lee, J., Duan, W., and Mattson, M. P. (2002) J. Neurochem. 82 1367-1375 [DOI] [PubMed] [Google Scholar]
- 7.Lee, J., Seroogy, K. B., and Mattson, M. P. (2002) J. Neurochem. 80 539-547 [DOI] [PubMed] [Google Scholar]
- 8.Parent, J. M. (2007) Prog. Brain Res. 163 529-817 [DOI] [PubMed] [Google Scholar]
- 9.Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S., and Lowenstein, D. H. (1997) J. Neurosci. 17 3727-3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun, D., McGinn, M. J., Zhou, Z., Harvey, H. B., Bullock, M. R., and Colello, R. J. (2007) Exp. Neurol. 204 264-272 [DOI] [PubMed] [Google Scholar]
- 11.Kempermann, G., Kuhn, H. G., and Gage, F. H. (1997) Nature 386 493-495 [DOI] [PubMed] [Google Scholar]
- 12.Nilsson, M., Perfilieva, E., Johansson, U., Orwar, O., and Eriksson, P. S. (1999) J. Neurobiol. 39 569-578 [DOI] [PubMed] [Google Scholar]
- 13.Lodha, R., and Bagga, A. (2000) Ann. Acad. Med. Singapore 29 37-41 [PubMed] [Google Scholar]
- 14.Huang, M. T., Lysz, T., Ferraro, T., Abidi, T. F., Laskin, J. D., and Conney, A. H. (1991) Cancer Res. 51 813-819 [PubMed] [Google Scholar]
- 15.Ruby, A. J., Kuttan, G., Babu, K. D., Rajasekharan, K. N., and Kuttan, R. (1995) Cancer Lett. 94 79-83 [DOI] [PubMed] [Google Scholar]
- 16.Shishodia, S., Sethi, G., and Aggarwal, B. B. (2005) Ann. N. Y. Acad. Sci. 1056 206-217 [DOI] [PubMed] [Google Scholar]
- 17.Chen, H., Zhang, Z. S., Zhang, Y. L., and Zhou, D. Y. (1999) Anticancer Res. 19 3675-3680 [PubMed] [Google Scholar]
- 18.Chuang, S. E., Kuo, M. L., Hsu, C. H., Chen, C. R., Lin, J. K., Lai, G. M., Hsieh, C. Y., and Cheng, A. L. (2000) Carcinogenesis 21 331-335 [DOI] [PubMed] [Google Scholar]
- 19.Rao, C. V., Rivenson, A., Simi, B., and Reddy, B. S. (1995) Ann. N. Y. Acad. Sci. 768 201-204 [DOI] [PubMed] [Google Scholar]
- 20.Calabrese, V., Butterfield, D. A., and Stella, A. M. (2003) Ital. J. Biochem. 52 177-181 [PubMed] [Google Scholar]
- 21.Lim, G. P., Chu, T., Yang, F., Beech, W., Frautschy, S. A., and Cole, G. M. (2001) J. Neurosci. 21 8370-8377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, F., Lim, G. P., Begum, A. N., Ubeda, O. J., Simmons, M. R., Ambegaokar, S. S., Chen, P. P., Kayed, R., Glabe, C. G., Frautschy, S. A., and Cole, G. M. (2005) J. Biol. Chem. 280 5892-5901 [DOI] [PubMed] [Google Scholar]
- 23.Thiyagarajan, M., and Sharma, S. S. (2004) Life Sci. 74 969-985 [DOI] [PubMed] [Google Scholar]
- 24.Sumanont, Y., Murakami, Y., Tohda, M., Vajragupta, O., Watanabe, H., and Matsumoto, K. (2006) Life Sci. 78 1884-1891 [DOI] [PubMed] [Google Scholar]
- 25.Wu, A., Ying, Z., and Gomez-Pinilla, F. (2006) Exp. Neurol. 197 309-317 [DOI] [PubMed] [Google Scholar]
- 26.Duvoix, A., Blasius, R., Delhalle, S., Schnekenburger, M., Morceau, F., Henry, E., Dicato, M., and Diederich, M. (2005) Cancer Lett. 223 181-190 [DOI] [PubMed] [Google Scholar]
- 27.Suh, H. W., Kang, S., and Kwon, K. S. (2007) Mol. Cell Biochem. 298 187-194 [DOI] [PubMed] [Google Scholar]
- 28.Lin, J. K. (2007) Adv. Exp. Med. Biol. 595 227-243 [DOI] [PubMed] [Google Scholar]
- 29.McNally, S. J., Harrison, E. M., Ross, J. A., Garden, O. J., and Wigmore, S. J. (2007) Int. J. Mol. Med. 19 165-172 [PubMed] [Google Scholar]
- 30.Scapagnini, G., Colombrita, C., Amadio, M., D'Agata, V., Arcelli, E., Sapienza, M., Quattrone, A., and Calabrese, V. (2006) Antioxid. Redox. Signal 8 395-403 [DOI] [PubMed] [Google Scholar]
- 31.Mattson, M. P., and Cheng, A. (2006) Trends Neurosci. 29 632-639 [DOI] [PubMed] [Google Scholar]
- 32.Menard, C., Hein, P., Paquin, A., Savelson, A., Yang, X. M., Lederfein, D., Barnabe-Heider, F., Mir, A. A., Sterneck, E., Peterson, A. C., Johnson, P. F., Vinson, C., and Miller, F. D. (2002) Neuron 36 597-610 [DOI] [PubMed] [Google Scholar]
- 33.Snyder, E. Y., Deitcher, D. L., Walsh, C., Arnold-Aldea, S., Hartwieg, E. A., and Cepko, C. L. (1992) Cell 68 33-51 [DOI] [PubMed] [Google Scholar]
- 34.Niles, L. P., Armstrong, K. J., Rincon Castro, L. M., Dao, C. V., Sharma, R., McMillan, C. R., Doering, L. C., and Kirkham, D. L. (2004) BMC Neurosci. 5 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang, Y., Ren, W., and Chen, F. (2006) Neuroreport 17 235-238 [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal, B. B., Banerjee, S., Bharadwaj, U., Sung, B., Shishodia, S., and Sethi, G. (2007) Biochem. Pharmacol. 73 1024-1032 [DOI] [PubMed] [Google Scholar]
- 37.Aoki, H., Takada, Y., Kondo, S., Sawaya, R., Aggarwal, B. B., and Kondo, Y. (2007) Mol. Pharmacol. 72 29-39 [DOI] [PubMed] [Google Scholar]
- 38.Al-Omar, F. A., Nagi, M. N., Abdulgadir, M. M., Al Joni, K. S., and Al-Majed, A. A. (2006) Neurochem. Res. 31 611-618 [DOI] [PubMed] [Google Scholar]
- 39.Zbarsky, V., Datla, K. P., Parkar, S., Rai, D. K., Aruoma, O. I., and Dexter, D. T. (2005) Free Radic. Res. 39 1119-1125 [DOI] [PubMed] [Google Scholar]
- 40.Zhu, Y. G., Chen, X. C., Chen, Z. Z., Zeng, Y. Q., Shi, G. B., Su, Y. H., and Peng, X. (2004) Acta Pharmacol. Sin. 25 1606-1612 [PubMed] [Google Scholar]
- 41.Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. (2001) Nature 410 372-376 [DOI] [PubMed] [Google Scholar]
- 42.Cameron, H. A., and McKay, R. D. (1999) Nat. Neurosci. 2 894-897 [DOI] [PubMed] [Google Scholar]
- 43.Haughey, N. J., Nath, A., Chan, S. L., Borchard, A. C., Rao, M. S., and Mattson, M. P. (2002) J. Neurochem. 83 1509-1524 [DOI] [PubMed] [Google Scholar]
- 44.Jackson-Guilford, J., Leander, J. D., and Nisenbaum, L. K. (2000) Neurosci. Lett. 293 91-94 [DOI] [PubMed] [Google Scholar]
- 45.Frautschy, S. A., Hu, W., Kim, P., Miller, S. A., Chu, T., Harris-White, M. E., and Cole, G. M. (2001) Neurobiol. Aging 22 993-1005 [DOI] [PubMed] [Google Scholar]
- 46.Baek, O. S., Kang, O. H., Choi, Y. A., Choi, S. C., Kim, T. H., Nah, Y. H., Kwon, D. Y., Kim, Y. K., Kim, Y. H., Bae, K. H., Lim, J. P., and Lee, Y. M. (2003) Clin. Chim. Acta 338 135-141 [DOI] [PubMed] [Google Scholar]
- 47.Piper, J. T., Singhal, S. S., Salameh, M. S., Torman, R. T., Awasthi, Y. C., and Awasthi, S. (1998) Int. J. Biochem. Cell Biol. 30 445-456 [DOI] [PubMed] [Google Scholar]
- 48.Zhang, M., Deng, C., Zheng, J., Xia, J., and Sheng, D. (2006) Int. Immunopharmacol. 6 1233-1242 [DOI] [PubMed] [Google Scholar]
- 49.Xiao, Z., Kong, Y., Yang, S., Li, M., Wen, J., and Li, L. (2007) Cell Res. 17 73-79 [DOI] [PubMed] [Google Scholar]
- 50.Kalluri, H. S., Eickstaedt, J., and Dempsey, R. J. (2007) Neurosci. Lett. 426 145-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng, A., Tang, H., Cai, J., Zhu, M., Zhang, X., Rao, M., and Mattson, M. P. (2004) Dev. Biol. 272 203-216 [DOI] [PubMed] [Google Scholar]
- 52.Cheng, A., Chan, S. L., Milhavet, O., Wang, S., and Mattson, M. P. (2001) J. Biol. Chem. 276 43320-43327 [DOI] [PubMed] [Google Scholar]
- 53.Lim, M. S., Nam, S. H., Kim, S. J., Kang, S. Y., Lee, Y. S., and Kang, K. S. (2007) Biochem. Biophys. Res. Commun. 357 903-909 [DOI] [PubMed] [Google Scholar]
- 54.Baron, W., Metz, B., Bansal, R., Hoekstra, D., and de Vries, H. (2000) Mol. Cell Neurosci. 15 314-329 [DOI] [PubMed] [Google Scholar]
- 55.Goel, A., Kunnumakkara, A. B., and Aggarwal, B. B. (2008) Biochem. Pharmacol. 75 787-809 [DOI] [PubMed] [Google Scholar]
- 56.Balogun, E., Hoque, M., Gong, P., Killeen, E., Green, C. J., Foresti, R., Alam, J., and Motterlini, R. (2003) Biochem. J. 371 887-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salvioli, S., Sikora, E., Cooper, E. L., and Franceschi, C. (2007) Evid. Based Complement Alternat. Med. 4 181-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu, Y., Ku, B., Cui, L., Li, X., Barish, P. A., Foster, T. C., and Ogle, W. O. (2007) Brain Res. 1162 9-18 [DOI] [PubMed] [Google Scholar]
- 59.Shin, H. J., Lee, J. Y., Son, E., Lee, D. H., Kim, H. J., Kang, S. S., Cho, G. J., Choi, W. S., and Roh, G. S. (2007) Neurosci. Lett. 416 49-54 [DOI] [PubMed] [Google Scholar]
- 60.Schmidt, H. D., and Duman, R. S. (2007) Behav. Pharmacol. 18 391-418 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.