Abstract
The hemoprotein cytochrome b5 (cyt b5) has been demonstrated to affect the kinetics of drug oxidation by the microsomal cytochromes P450. However, the mechanisms through which cyt b5 exerts these effects are variable and P450 isoform-dependent. While the effects of cyt b5 on the major drug metabolizing enzymes CYP2D6, CYP2E1, and CYP3A4 are well studied, fewer studies conducted over limited ranges of cyt b5 concentrations have been performed on CYP2C9. In the present study with CYP2C9, cyt b5 exerted complex actions upon P450 oxidative reactions by affecting the rate of metabolite formation, the consumption of NADPH by cytochrome P450 reductase, and uncoupling of the reaction cycle to hydrogen peroxide and water. Cytochrome b5 devoid of the heme moiety (apo-b5) exhibited similar effects as native cyt b5. All rates were highly dependent on the cyt b5 to CYP2C9 enzyme ratio suggesting that the amount of cyt b5 present in an in vitro incubation is an important factor that can impact the reliability of extrapolating in vitro generated data to predict the in vivo condition. The major effects of cyt b5 are hypothesized to result from a cyt b5 induced conformational change in CYP2C9 that results in an increased collision frequency between the iron-oxygen species (Cpd I) and the substrate, and a decrease in the oxidase activity. Together, these findings suggest that cyt b5 can alter multiple steps in the P450 catalytic cycle via complex interactions with P450 and P450 reductase.
The cytochrome P450 (CYP) enzyme family catalyzes the oxidation of a wide variety of structurally diverse compounds (Guengerich, 1997). In light of the large number of drug classes that interact with human CYPs, a thorough understanding of this enzyme system is necessary in developing therapeutic agents that can safely be administered in the treatment of diseases. Traditionally, characterization of the involvement of the major hepatic CYPs (CYP1A2, 2C9, 2C19, 2D6, 2E1 and 3A4) in drug metabolism is conducted using an array of in vitro methodologies including regression analysis with a panel of human liver microsomes (HLM), chemical and/or immuno-inhibition of the particular oxidative biotransformation, and metabolism by single recombinant CYP systems (rCYPs) (Wrighton, et al., 1993). Recent advances in recombinant DNA technology have allowed for significant improvements in evaluating the in vitro role of human CYPs and as a consequence, have increased the relative importance of recombinant CYPs as a complementary enzyme system to HLM (Crespi and Miller, 1999).
While the use of rCYPs has gained utility, there are still issues associated with relating observations generated from rCYP assays to results obtained from HLM. For example, several laboratories have reported the predicted contribution of CYP1A2 to drug biotransformation from rCYP to be substantially greater than that predicted from HLM (Rodrigues, 1999;Venkatakrishnan, et al., 2000). It has been postulated that differences between rCYPs and HLM may be attributed to differing expression levels of cytochrome P450 oxidoreductase (CPR) or cytochrome b5 (cyt b5) in each system. Moreover, Nakajima et al., demonstrated that the coexpression of recombinant CYPs with cyt b5 plays an important role in robustness of data when comparing results between rCYPs and HLM (Nakajima, et al., 2002). The hemoprotein cyt b5 transfers electrons for a wide array of reactions occurring in the endoplasmic reticulum and mitochondrial membranes (reviewed by Schenkman (Schenkman and Jansson, 2003) and Porter (Porter, 2002)). For instance, cyt b5, anchored by a C-terminal hydrophobic helix, interacts directly with the membrane-bound CYPs and CPR to alter the rate of P450 catalysis. The addition of cyt b5 to mixtures of CYPs and CPR results in varied effects on the activity of P450-mediated drug oxidation. There are reports of increases (Yamazaki, et al., 2002), decreases (Gruenke, et al., 1995;Morgan and Coon, 1984), or no effect on the rate of drug metabolism, and changes in kinetic profiles (Jushchyshyn, et al., 2005) induced by cyt b5, all of which appear to be P450 isoform- and substrate-dependent. Any correlation between reconstituted systems, and more complex systems, such as microsomes requires an understanding of how the different constituents in the system interact. Thus, the effects of different ratios of P450, CPR, and cyt b5 must be understood for each of the major drug metabolizing enzymes.
Cyt b5 can impact the rates of substrate oxidation at a number of steps in the catalytic cycle (Scheme 1). It has been shown to enhance electron transfer, resulting in increased NADPH consumption, and presumably production of the iron-oxygen species, also known as Cpd I. This direct effect of cyt b5 in enhancing electron transfer increases the activity of CYP2E1 and CYP3A4. Cytochrome b5-induced conformational effects on the CYP3A4 protein structure are also thought to stimulate CYP3A4 in a manner independent of the electron transfer process (Perret and Pompon, 1998). Interestingly, cyt b5 has no effect on the major drug metabolizing enzyme 2D6 (Yamazaki, et al., 2002). While the cyt b5 effect has been thoroughly studied for CYP2E1 and CYP3A4 only two reports of the effects of cyt b5 on the major drug metabolizing enzyme CYP2C9 appear in the literature (Yamazaki, et al., 1997;Yamazaki, et al., 2002). These investigators reported that both cyt b5 and apo-b5 stimulated the metabolism of tolbutamide and (S)-warfarin by 2- and 5-fold, respectively. In these studies, the ratio of cyt b5 to P450 was varied from 0 to 2, with an apparent saturation of cyt b5 binding (Yamazaki, et al., 2002). Effects due to changes in buffer, ionic strength and the influence of other P450 enzymes on activity were observed, but the mechanism of cyt b5 stimulation of CYP2C9 was not apparent.
To explore the mechanism(s) of cyt b5 stimulation of CYP2C9, the effect of a wide range of cyt b5 concentrations on oxidation of three [(S)-flurbiprofen, diclofenac and (S)-warfarin] CYP2C9 substrates and P450 cycle stoichiometry was examined. In addition, studies were conducted with apo-b5 to determine if the cyt b5 effects were solely due to provision of electrons or whether cyt b5-induced conformational changes might play a role in altering CYP2C9 activity.
Materials and Methods
Materials
All biochemicals and reagents were from Sigma-Aldrich/Fluka (St. Louis, MO). The 4′-OH metabolite of diclofenac was purchased from BD Gentest (Bedford, MA). (S)-warfarin and the 7-OH-warfarin metabolite were gifts from Professor William Trager at the University of Washington. (S)-flurbiprofen and 4′-OH flurbiprofen were gifts from the former Pharmacia Corporation (Kalamazoo, MI).
Enzymes
Full length, recombinant human CYP2C9 with the N-terminal sequence MALLLAVF was expressed and purified as before (Locuson, et al., 2006). Cytochrome P450 reductase (CPR) from rat was expressed and purified from E. coli as described previously (Hanna, et al., 1998). Detergent solubilized CYP2C9 and CPR underwent chromatography over hydroxyapatite and dialysis to help eliminate as much detergent as possible before freezing the enzymes in buffer containing 20 % glycerol. Purified recombinant human cyt b5 was purchased from Invitrogen (Carlsbad, CA). Apo-b5 was a gift from Dr. Monica Jushchyshun (Pfizer Global Research and Development, St. Louis Laboratories) and was produced from the same Invitrogen human cyt b5 by the method of Yamazaki et al (Yamazaki, et al., 1996a). The concentration of apo-b5 was determined by the BCA assay with bovine serum albumin as the standard (Pierce Biotechnology, Rockford, IL).
Reconstitution conditions
Enzymes were reconstituted by adding P450 to CPR and incubating the mixture on ice for at least 5 min, followed by addition of lipid. Liposomes formed by extrusion of 1,2-dilauroyl-sn-glycero-3-phosphocholine were added to gain optimal diclofenac 4′-hydroxylation activity indicating successful reconstitution of 2C9 with CPR (∼ 8-10 nmol/min/nmol CYP2C9 at 37 °C, no cyt b5). Optimal activity was achieved between 0.5 and 2.0 μg 1,2-dilauroyl-sn-glycero-3-phosphocholine/pmol CYP2C9 with the latter being used to ensure an adequate supply of lipid over the wide range of P450-CPR-cyt b5 ratios employed. Higher amounts of lipid, while more physiologically relevant, decreased activity presumably by limiting the incorporation of enzymes into the same liposome. Through use of a fixed pore size polycarbonate membrane (200 nm), the diameter of the bilayer liposomes average 200 nm after extrusion (Avestin, Ottawa, Ontario). After 5 additional min, cyt b5 was then added. Two hour enzyme and lipid reconstitution intervals on ice or room temperature only increased the activity of the resulting liposome-enzyme mixtures by < 15 %. The widest ranges of CPR:P450 and cyt b5:P450 ratios used were 0.2 – 16 and 0.1 – 50, respectively, unless otherwise noted. Incubations where cyt b5 and lipid were added before CPR were also conducted at suboptimal CPR concentrations (2:1 CYP2C9:CPR) for comparison.
Activity assays
All enzyme assays were performed at 37 °C with 10-20 pmol of CYP2C9 with a range of limiting, equal, or saturating amounts of CPR and cyt b5. Reactions were carried out either in 1.5 mL microcentrifuge tubes in a temperature controlled water bath or in a quartz cuvette in a peltier-equipped UV-vis spectrophotometer. After five min of temperature equilibration, reactions were initiated with 1 mM NADPH unless otherwise noted. Further details regarding assays have been previously described (Locuson, et al., 2006).
Diclofenac
Diclofenac is hydroxylated at its 4′-position by CYP2C9. Saturating concentrations of diclofenac were used (200 μM), and reactions were allowed to proceed for 15 min. A solution of acetonitrile (0.1 mL) containing 7-ethoxycoumarin (1 nmol, retention time = 6.5 min) as an internal standard was used to stop the reactions. 4′-OH diclofenac (retention time = 7.8 min) was then separated by HPLC and quantitated by UV absorption at 282 nm. The mobile phase consisted of 30 % methanol and 70 % of a 30 % acetonitrile solution containing 1 mM perchloric acid. A linear gradient to 80 % methanol over 10 min was started one min after injection to carry out separation. This method is based on that used by Haining et al (Haining, et al., 1999).
(S)-warfarin
The formation of 7-OH-warfarin, the primary hydroxylation product of (S)-warfarin by CYP2C9, was monitored by the fluorescence method exactly as described by Lang and Böcker (Lang and Bocker, 1995). Incubations were carried out with 100 μM (S)-warfarin for 45 min, stopped with 5 μL of 70 % perchloric acid, and spiked with 20 pmol of internal standard, 7-ethoxycoumarin.
(S)-flurbiprofen
Hydroxylation of (S)-flurbiprofen at the 4′ position was quantitated via fluorescence exactly as described (Tracy, et al., 2002).
H2O2
Hydrogen peroxide formation was monitored with freshly prepared xylenol orange reagent described by Jiang et al. (Jiang, et al., 1990). Reactions were carried out exactly as described (Locuson, et al., 2006). In all cases, separate control reactions were run for each enzyme ratio, but in the absence of substrate. Any background level of H2O2 formed in the absence of substrate was subtracted from the H2O2 formation measured in the presence of substrate.
NADPH
The use of NADPH by the enzyme system with the substrate diclofenac was measured by the change in absorbance at 340 nm (ε = 6.23 mM−1•cm−1) as a function of time. Reactions were initiated with 0.3 mM NADPH and typically run for 3 min. Data points taken 10 seconds apart were used to calculate the moles of NADPH consumed per min.
NADPH measurements with the substrates (S)-flurbiprofen and (S)-warfarin were conducted using a coupled enzyme system, which increased sensitivity and required much less enzyme (10-20 pmol P450) for these lower turnover substrates. Reactions (0.2 mL each) containing 1 U of isocitrate dehydrogenase and 10 mM isocitrate were initiated with 0.1 mM NADPH, allowed to proceed for 30 min, and quenched with 20 μL of 70 % perchloric acid (Gruenke, et al., 1995). Next, 0.1 mL of 1 mM 2,4-dinitrophenylhydrazine/1 M HCl solution was added and incubated at room temperature for one hour. Formation of the hydrazone of α-ketoglutarate was monitored spectroscopically at 390 nm to ensure completion of the reaction. Next, 0.3 mL of 10 % NaOH was added and the samples monitored immediately at 515 nm along with standard curve samples. This wavelength was chosen, versus 440 nm, due to its wider linear range (Anthon and Barrett, 2003). Standard curves were prepared using α-ketoglutarate at 5-100 μM and processed at the same time as enzyme reaction samples.
Heme incorporation by apo-cytochrome b5
An experiment was carried out to ensure that apo-b5 was not forming its native heme-bound state by scavenging heme from CYP2C9. Native cyt b5 (1 μM) and apo-b5 (1 μM) were incubated separately with CYP2C9 (1 μM) for one hour under identical buffer, lipid, and temperature conditions used for the drug metabolism reactions outlined above. Hence, the duration of this incubation was at least three times longer than drug-containing reactions that included diclofenac or (S)-flurbiprofen. At this time, the samples were reduced with Na2S204 and subjected to an absorbance wavelength scan in split beam mode. An identical sample containing reduced CYP2C9 but lacking cyt b5 was used in the reference cell as described previously (Yamazaki, et al., 2001). No significant apo-b5 to native cyt b5 transition was noted (data not shown).
Equilibrium binding of CYP2C9 redox partners
The binding (KS values) of CPR and cyt b5 to CYP2C9 were assessed by their ability to influence the absorbance spectrum of CYP2C9. Although this technique is not amenable to all P450 isoforms, both cyt b5 and CPR were able to induce measurable changes in the spin state of CYP2C9 in an analogous manner as was observed with rabbit CYP2B4 (Tamburini, et al., 1985). Experiments were conducted with an Olis upgraded Aminco DW-2000 UV/Vis spectrophotometer (Olis, Bogart, GA) operating in split beam mode as described by Tamburini (Tamburini, et al., 1985). The sample cuvette contained 0.2 μM CYP2C9 in 50 mM potassium phosphate buffer (pH 7.4) and 20 % glycerol. Extruded 1,2-dilauroyl-sn-glycero-3-phosphocholine (100 μg) was added, though it provided no discernable differences in the total spectroscopic signal change or in KS values. The reference cuvette contained only buffer and glycerol. Next, either concentrated CPR (0.001 – 0.7 μM) or cyt b5 (0.001 – 1.8 μM) was titrated into both sample and reference cuvettes and scanned from 340 – 500 nm to monitor the peak heights of the low and high spin Soret bands (390 and 418 nm, respectively). The differences between the two peaks in the absolute spectra were used for calculating the fraction of CYP2C9 complexed to either CPR or cyt b5 based on the following rationale. First, the change in the low spin peak area as determined by gaussian curve fitting resulted in estimation of similar KS values as were obtained during data fitting to those estimated by calculating the differences in peak height. Second, by adding the P450 redox partners to both cuvettes, the cyt b5 and CPR signals that absorb at similar wavelengths as the P450 heme are kept to a minimum. Experiments were conducted at 30 °C and samples allowed to sit at least 20 min after each addition of enzyme to reach equilibrium, as confirmed by periodic sample scanning.
KS values for CPR and cyt b5 were determined with DynaFit (Kuzmic, 1996) using floating molar response coefficients, the total measured P450 concentration (Omura and Sato, 1964), and an equilibrium expression for CPR binding:
or, two distinct cyt b5 binding events as suggested by the observed two apparent phases:
Addition of substrate to CPR titrations abolished the ability to detect CPR binding, but CPR binding could still be detected in the presence of an equimolar mixture of P450+cyt b5 and for cyt b5 with P450+CPR.
Results
Reconstitution
The reconstitution of membrane-bound P450s into liposomes has previously been optimized with respect to buffers, various ions, and mixtures of lipids (Yamazaki, et al., 1997). In our hands, using liposomes in inadequate amounts (< 0.5 μg/pmol P450) led to suboptimal activity while the use of excessive levels (> 2.0 μg/pmol P450) of liposomes in effect diluted the enzymes so that higher levels of CPR were required to obtain the same activity with a given concentration of P450 (data not shown). The average sized liposome was 200 nm in diameter based on the extrusion membrane and the lipid/protein ratio was 40 (w/w), which is on the order of that typically used in the reconstitution of membrane-bound proteins to achieve activity and ‘infinitely dilute’ any residual detergent (Ollivon, et al., 2000). A wide range of total enzyme concentrations were tested as described in the Experimental section.
To determine if the order of addition changed the results, reconstitution was also carried out in the reverse order of that described above. In this case P450 was added to cyt b5, followed by addition of lipid, and then a limiting amount of CPR (P450:CPR = 2) to allow cyt b5 to better interact with CYP2C9. Cyt b5 and apo-b5 stimulated (S)-flurbiprofen hydroxylation by CYP2C9 to the same degree regardless of the order of addition of the enzymes (data not shown).
Cytochrome b5 effects on CYP2C9 substrate oxidation
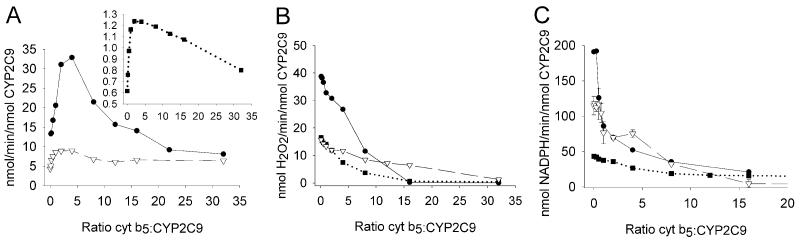
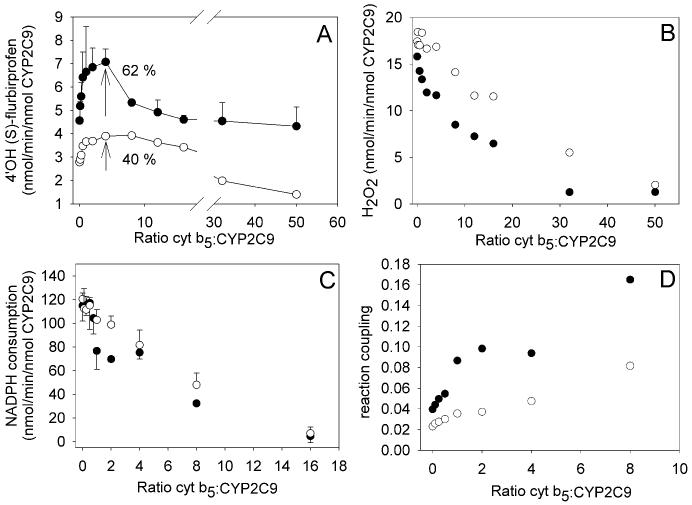
A wide range of limiting and saturating cyt b5 concentrations were added to the reconstituted CYP2C9-CPR-lipid enzyme mixture. Initially, CPR was held constant at a ratio of 2:1 CPR:CYP2C9 when comparing the substrates diclofenac, (S)-warfarin, and (S)-flurbiprofen (Fig. 2). The rate of oxidation of diclofenac increased to a maximum around 250% relative to the rate with no cyt b5. The maximum was reached when the ratio of cyt b5 to P450 was four, but decreased when the ratio exceeded ∼4 (Fig. 2a). The rate of oxidation of (S)-flurbiprofen (Fig. 2a) and (S)-warfarin (Fig. 2a, inset) hydroxylation was stimulated approximately 100%, at an enzyme ratio of 4, and decreased with higher ratios of cyt b5/P450. At a ratio of sixteen molar equivalents of cyt b5 to P450 the level of metabolite formation from all substrates declined to levels similar to incubations without cyt b5.
Fig. 2.
Cyt b5 concentration effects on the catalysis of liposome reconstituted CYP2C9-CPR with drugs diclofenac (●) (200 μM), (S)-flurbiprofen (▽) (100 μM), and (S)-warfarin (■) (100 μM). (A) drug metabolite formation rate. (B) H2O2 formation rate. (C) NADPH consumption rate. Data points represent the average of duplicate determinations except when error bars are present (triplicate ± standard deviation).
Cytochrome b5 effects on CYP2C9 Hydrogen Peroxide Formation
At the ratio of cyt b5/P450 that gave maximum product, the formation of hydrogen peroxide decreased 31%, 27%, and 55% for diclofenac, (S)-flurbiprofen, and (S)-warfarin, respectively, as compared to the absence of cyt b5. At ratios of cyt b5/P450 ≥ 16 (Fig. 2b), H2O2 formation dropped to < 0.2 nmol/min/nmol P450, which was near the detection limit of the assay.
Cytochrome b5 effects on CYP2C9 NADPH Consumption
The rates of NADPH consumption by CPR in the reconstituted enzyme system were also measured at different cyt b5 ratios (Fig. 2c). These values indicate both the frequency that the catalytic cycle is initiated and the amount of “excess” water formed by 2 electron reduction of the ferryl-oxo intermediate, also called Cpd I (Scheme 1). Across all concentrations of cyt b5, the amount of NADPH consumed (diclofenac > (S)-flurbiprofen > (S)-warfarin) followed the same order as observed for the H2O2 formation rates. Interestingly, cyt b5 decreased NADPH oxidation with all three substrates, even while metabolite formation was stimulated.
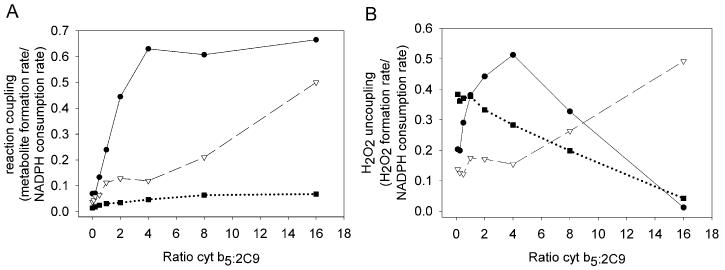
Cytochrome b5 effects on coupling of electrons to metabolite formation
The presence of cyt b5 exerts substantial effects on the coupling (e.g, the moles of metabolite formed divided by the moles of NADPH consumed) for all three substrates (Fig. 3a). As the ratio of cyt b5 to 2C9 was increased from zero to four, the fraction of NADPH used to form the metabolite of diclofenac increases from 7.0 to 63.0 %. For (S)-flurbiprofen, and (S)-warfarin, the fraction of NADPH used to product formed, increased from 3.8 to 11.9 %, and from 1.4 to 4.7 %, respectively. Since cyt b5 decreased oxidase activity, which uses two equivalents of NADPH, this suggests that the increase in coupling was more a result of reduced NADPH consumption than an increase in substrate oxidation (Fig. 2a and c). At higher ratios of cyt b5 to P450, a plateau in the ratio of product to NADPH consumption was observed with diclofenac and warfarin. In the case of flurbiprofen, NADPH consumption appeared to level off and then increase. However, the levels of NADPH consumed at the two highest concentrations of cyt b5 was very low and thus, computing a ratio with such a small number exaggerates the error in this measurement.
Fig. 3.
Coupling and uncoupling changes in CYP2C9 catalysis with the drugs diclofenac (●) (200 μM), (S)-flurbiprofen (▽) (100 μM), and (S)-warfarin (■) (100 μM) induced by alternate cyt b5 concentrations. (A) reaction coupling to drug metabolite formation (metabolite formation rate/NADPH consumption rate). (B) reaction uncoupling to H2O2 (H2O2 formation rate/NADPH consumption rate).
Changes in uncoupling to H2O2 (H2O2 formed/NADPH consumed) (Fig. 3b) were variable but appeared to increase or remain the same at ratios of cyt b5/P450 below 2-4 for all substrates. The degree of uncoupling then decreased at higher cyt b5/P450 ratios, except with flurbiprofen. As noted above, the results for flurbiprofen at high ratios are most likely associated with error in the denominator (NADPH used) resulting from assay sensitivity limitations due to the dramatic decrease in NADPH consumption.
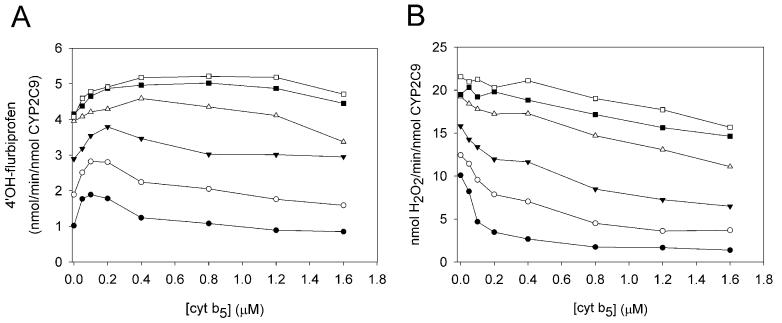
Effect of the CPR – cyt b5 enzyme ratio
All the measurements described above were conducted at a fixed (2:1) ratio of CPR to P450. To further assess the combined effect of CPR and cyt b5 concentrations on CYP2C9 metabolism, (S)-flurbiprofen was used as the substrate and ratios of CPR/CYP2C9 and cyt b5/CYP2C9 were varied (Fig. 4). (S)-Flurbiprofen was chosen as the substrate because its metabolite could be detected by a highly sensitive fluorescence HPLC assay even at low CPR concentrations. Formation of 4′OH-flurbiprofen and the amount of H2O2 formed as a shunt product of this reaction were determined. The degree of maximal stimulation provided by cyt b5 (four eq) was for the most part independent of the CPR concentrations used and ranged from 21 to 27 % (Fig. 4a). For H2O2 production, higher CPR concentrations inhibited the ability of cyt b5 to diminish uncoupling to H2O2 (Fig. 4b). In the presence of 0.5 eq of CPR to P450, cyt b5 reduced H2O2 formation 7.4-fold and in the presence of twelve eq of CPR H2O2 formation was reduced only 1.4-fold.
Fig. 4.
Combined effects of CPR and cyt b5 concentration on the metabolism of (S)-flurbiprofen to 4′-OH-flurbiprofen and H2O2 formed as a result of catalytic uncoupling (CYP2C9 = 100 nM). (A) rate of 4′-OH-flurbiprofen formation. (B) rate of H2O2 formation. CPR concentrations were as follows: 50 nM (●), 100 nM (○), 200 nM (■), 400 nM (□), 800 nM (▼), 1200 nM (△).
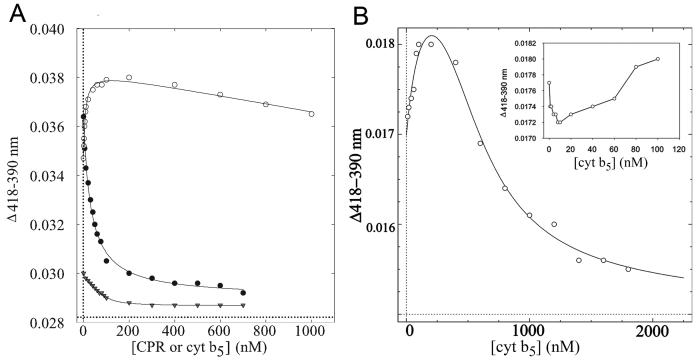
Equilibrium binding of CPR and cyt b5 to P450
Binding affinity constant values (KS) for CPR and cyt b5 were determined based on the ability of CPR and cyt b5 to perturb the heme iron spin state equilibrium of CYP2C9 (Fig. 5). CPR increased the population of high spin heme while lower concentrations of cyt b5 increased the fraction of low spin heme, but the effect reversed at cyt b5 concentrations equimolar to CYP2C9. Table 1 lists the determined KS values. Even under saturating CPR or cyt b5 conditions, the decrease in low spin P450 was modest (< 10 %) as determined from gaussian curve fitting of the absolute spectra (Locuson, et al., 2006). However, the effects were reproducible and highly dependent on the amount of added CPR or cyt b5. The affinity of CPR was not altered by the presence of cyt b5 (Table 1). Conversely, the cyt b5 binding profile was more complex in the presence of CPR (Fig. 5b) and fitting did not allow estimation of KS for the first phase, but the lower affinity second and third phases could be fit using an equilibrium model for two, distinct cyt b5 binding events (Table 1).
Fig. 5.
Binding of CPR and cyt b5 to CYP2C9 (200 nM) as measured by the difference between the peak heights of the high spin (HS) and low spin (LS) heme iron components in P450 absolute spectra. (A) CPR binding (●), cyt b5 binding (○), and CPR binding with equimolar CYP2C9/cyt b5 (▼). (B) cyt b5 binding with equimolar CYP2C9/CPR (low cyt b5 concentrations shown in inset for clarity).
Table 1.
Measured binding constants of CYP2C9 complexes.
| complex | KS (nM)a |
|---|---|
| CYP2C9 + CPR | 32.8 ± 0.2 |
| CYP2C9 + b5 | 8.1 ± 0.9 |
| CYP2C9•CPR + cyt b5 | 2nd phase ∼ 221 |
| 3rd phase ∼ 794 | |
| CPR + cyt b5 | NDb |
| CYP2C9•cyt b5 + CPR | 25.6 ± 0.7c |
Errors represent the error of the parameter estimate derived from non-linear regression
Not determined
Determined with cyt b5 equimolar to CYP2C9, which was at or near saturating levels according to the spectral signal
Coupling changes induced by apo-b5
Apo-b5 lacks the heme prosthetic group normally liganded to two histidine residues at the heme iron. Therefore, while apo-b5 could influence electron transfer from CPR to P450, it most likely is not capable of existing as a lone, reduced intermediate. In the present studies, apo-b5 exerted relatively similar effects as native cyt b5 as evidenced by its ability to increase (S)-flurbiprofen metabolite formation and decrease H2O2 formation (Fig. 6a and b). Furthermore, control studies (data not shown) demonstrated that apo-b5 was not converted to its native heme-bound state on the time scale used for these studies. Optimal stimulation of metabolite formation was observed at four eq of apo b5 to CYP2C9 with an increase in metabolite production of 40 % in the presence of apo-b5. This was the same ratio of b5/P450 that resulted in maximal stimulation (62 %) with native cyt b5. H2O2 formation was 44 % higher, on average, with apob5, but apo-b5 clearly remained effective at reducing H2O2 formation when present in equimolar or higher levels than P450. Reaction coupling (metabolite/NADPH ratio) was also increased 2.1-fold with apo-b5 at four eq of apo-b5 compared to 2.4-fold with native cyt b5 (Fig. 6d).
Fig. 6.
Comparison between the activities of native cyt b5 (●) and apo-b5 (○) on the metabolism of (S)-flurbiprofen with a 1:2 ratio of CYP2C9:CPR. (A) rate of (S)-flurbiprofen metabolite formation. (B) rate of H2O2 formation. (C) rate of NADPH consumption. (D) calculation of reaction coupling to metabolite formation (metabolite formation rate/NADPH consumption rate). Data points represent the average of duplicate determinations except when error bars are present (triplicate ± standard deviation). Experiments with native or apo-b5 were conducted on different days resulting in product levels being slightly offset.
Discussion
With the recognition that metabolic properties can play a major role in the success of a new drug, the focus on identifying the enzymes responsible for the metabolism of a potential drug has increased. In early drug discovery, in vitro systems are used including human liver microsomes, microsomes containing expressed enzymes (e.g., Baculosomes®), and occasionally, reconstituted purified enzymes. The hope is that in vitro systems can be predictive of human in vivo metabolism. While purified and reconstituted systems provide definitive evidence that a given P450 enzyme can metabolize a drug, the artificial nature of the reconstitution system can lead to quantitative differences. Several studies have examined the effects of varying the concentration of various cellular components on the activity of P450 enzymes in vesicles. Cyt b5 has been shown to activate at least 16 different P450 isoforms (Porter, 2002), including CYP3A4, 2E1 and 2C9. The activation of cyt b5 on CYP3A4, while controversial, has been extensively studied (Perret and Pompon, 1998;Guryev, et al., 2001;Yamazaki, et al., 1996a), and appears to involve multiple mechanisms. The primary effect of cyt b5 on CYP2E1, is again activation, and appears to involve a single mechanism - enhanced electron transfer from cyt b5 to CYP2E1 (Yamazaki, et al., 1996b;Yamazaki, et al., 2002). One report has concluded that CYP2D6 is relatively unique in that cyt b5 has no effect on the rates of metabolism by this enzyme (Yamazaki, et al., 2002). Relative to the other major metabolic enzymes, fewer studies of cyt b5 effects on CYP2C9 have been reported. These studies have used relatively low ratios of cyt b5 to P450, and have reported hyperbolic activation (Yamazaki, et al., 1997;Gorsky and Coon, 1986;Yamazaki, et al., 2001). Given the importance of CYP2C9 in drug metabolism, a thorough understanding of the role of cyt b5 on the rates of metabolism is needed to optimize reconstituted in vitro systems, for use in drug metabolism studies.
The qualitative changes observed at different cyt b5 concentrations with CYP2C9 are similar for each of the three substrates. Optimum product formation was observed at 4 equivalents of cyt b5, hydrogen peroxide production decreased with increasing cyt b5/CYP2C9 ratios, and an apparent decrease in NADPH dependent oxidase activity was noted with increasing cyt b5/CYP2C9 ratios. Oxidase activity results from 4 electron reduction of oxygen to two waters by two equivalents of NADPH. Thus, a decrease in oxidase activity results in an increase in coupling of NADPH to product formation (a process that only requires one equivalent of NADPH). Spectroscopic measurements suggested that cyt b5 and CPR do not share a single common site of interaction with CYP2C9, although some degree of competitive interaction was observed at high cyt b5 concentrations. Finally, using flurbiprofen as a model substrate, experiments with apo-b5 (i.e., heme removed) still resulted in increases in coupling to a level almost 70% that observed with native cyt b5. Control experiments demonstrated that heme transfer to the apo-b5 (from P450) did not occur in the time-scale used in these experiments, suggesting that the major role for cyt b5 is not to act as an electron sink.
Given these observations, a mechanism for the cyt b5 effect on CYP2C9 can be hypothesized. It is proposed that through protein-protein interactions, binding of cyt b5 causes CYP2C9 to undergo a conformational change that decreases the active-site volume. This would limit the number of free waters in the active-site, decreasing protonation of the oxygen proximal to the iron in the iron-peroxo intermediate (also known as Cpd 0) and subsequently reduce hydrogen peroxide release (Scheme 1). The ordered water molecules associated with the I-helix water channel are thus better able to protonate the distal oxygen of the iron-peroxo intermediate resulting in formation of the iron-oxo species, Cpd I. This mechanism would explain the difference in peroxide and product formation observed with increased cyt b5 levels, but not the increase in coupling between NADPH and product formation. However, a decrease in active-site volume would also increase collision frequency between Cpd I and the substrate, increasing the amount of product formed per Cpd I oxygen produced. An increase in collision frequency decreases the lifetime of Cpd I, and the probability that it will be reduced by another two electrons to form a second molecule of water. The resulting decrease in oxidase activity would account for the greater change in NADPH consumption, relative to product formation. This line of reasoning is also consistent with the observation that when sufficient levels of hydrogen peroxide, in the absence of NADPH, are present to support catalysis by shunting into the P450 catalytic cycle, cyt b5 can still stimulate metabolite formation (Kumar, et al., 2005). At very high concentrations of cyt b5, competition between CPR and cyt b5 at a purported common site would result in a decrease in electron transfer from NADPH, as well as formation of products, including hydrogen peroxide and water.
An explanation of the differences in CYP2C9 function observed between apo-b5 and the native cyt b5 requires a second (heme dependent) mechanism, independent of the decreased active-site volume. It is possible that the conformation of apo-b5 is affected by removal of the heme, and that apo-b5 does not induce the same extent of conformational change as the holo-enzyme. This would imply that the binding affinity of apo-b5 to CYP2C9 is similar to that of native cyt b5 (Figure 6) but that the effect of this binding is attenuated. Alternatively, the difference could be due to native cyt b5 acting as an electron sink, decreasing the oxidase activity by slowing the delivery of the third and four electrons and thus increasing the life-time of Cpd I.
Since earlier studies on cyt b5-CYP2C9 interactions employed a narrow range of cyt b5 to 2C9 ratios (0 to 2), it is of interest to understand the range of ratios observed in human livers. The concentration of cyt b5 in human liver microsomes has been reported to be 374 pmol/mg of protein, which gives a ratio of cyt b5 to CYP proteins in human microsomes that varies between 1-200 depending on the P450 enzyme (Venkatakrishnan, et al., 2000). A positive correlation was observed for metabolic activity versus the ratio of cyt b5 to P450 3A4, 2B6, and 1A2 activity suggesting that in microsomes, the concentration of cyt b5 may be an important determinant of activity. The cyt b5 effects on CYP2C9 were not determined in the abovementioned study (Venkatakrishnan, et al., 2000). However, based on the variation in cyt b5 to P450 ratios for other enzymes in human liver, it is likely that the full range of cyt b5 to 2C9 ratios presented herein would be observed in human microsomes, and in vivo.
The overall rates of metabolism of a compound by any P450 is determined by a complex interplay between the rate of the first two electrons transferred to generate Cpd 0, a competition between protonation of Cpd 0 to give Cpd I or the release of hydrogen peroxide, and a second competition between oxidation of substrate and reduction of water to form a second molecule of water (oxidase activity) (Scheme 1). These branched pathways are likely responsible for the weak correlation between NADPH consumption, and product formation. The pronounced effect of cyt b5 on electron transfer and shunting of the reaction to each of the branch points demonstrates the importance of the levels of each component in the reconstituted system in determining the amounts of each product formed. It can be speculated that the organism's desire to balance the use of reducing equivalents versus xenobiotic detoxification should lead to a system in vivo producing the highest ratio of substrate oxidation to NADPH consumption. The results shown in Figure 2a indicate that this optimum metabolic condition requires 4-5 equivalents of cyt b5 for all three of the substrates, a value higher than normally used in vitro. Furthermore, given these results and the potential differences in interactions in a vesicle relative to intact endoplasmic reticulum, it is not clear that a simple reproduction with in vitro systems of the ratios of cyt b5, CPR and P450 observed in vivo will accurately reflect the interactions occurring in vivo. The mechanism we propose may not be specific to cyt b5, or even redox active proteins, as protein-protein interactions may occur with a number of proteins in the cellular matrix. In fact, with the high concentration of proteins in cells in vivo the apparent conformational effect of cyt b5 on CYP2C9 may be a general effect that can be mediated by any cellular protein, including other P450 proteins as reported and reviewed by Backes and colleagues (Backes and Kelley, 2003).
In conclusion, cyt b5 exhibited concentration-dependent effects on CYP2C9-mediated drug oxidation. The ability of cyt b5 to greatly increase P450 coupling, at enzyme ratios found in liver microsomes where cyt b5 exceeds the levels of the human P450s, has not previously been reported. Though reconstituted P450 systems will not exactly reflect the membrane environment of the endoplasmic reticulum, it is important to understand the effects of physiological concentrations on cyt b5 on P450 function. The majority of these effects can be explained by a cyt b5-induced conformational change in CYP2C9 resulting in a decrease in active-site volume leading to the exclusion of water from the active-site and an increase in collisions between the substrate and the active-oxygen species.
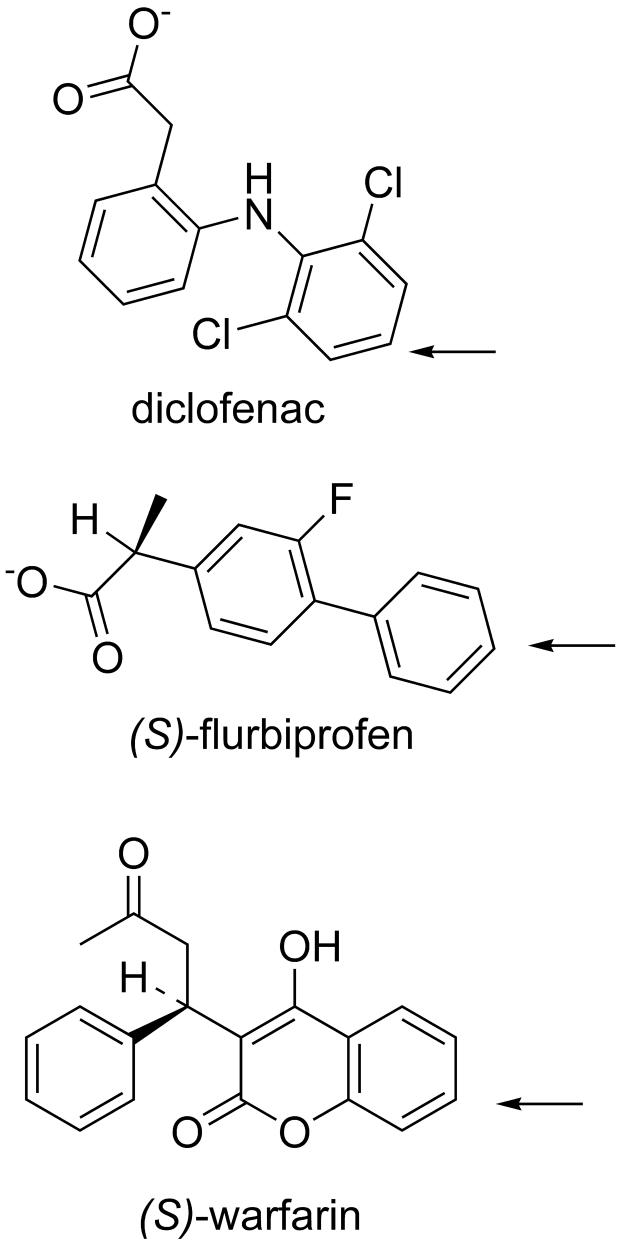
Fig. 1.
Stuctures of CYP2C9 substrates diclofenac, (S)-flurbiprofen, and (S)-warfarin with arrows denoting their sites of hydroxylation.
Acknowledgement
We thank Drs. Lisa M. Gloss, Petr Kuzmic, and Ronald A. Siegel for technical assistance and Dr. Monica Jushchyshun for providing the apo-cytochrome b5. In addition, we are grateful for resources from the University of Minnesota Supercomputing Institute.
*Financial support: This work was supported in part by a grant from the National Institutes of Health to T.S.T. (GM063215) and J.P.J. (ES009122).
Non-standard abbreviations
- P450
cytochrome P450
- CPR or reductase
cytochrome P450 oxidoreductase
- cyt b5
cytochrome b5
- apo-b5
apo-cytochrome b5
- rCYP
recombinant cytochrome P450
- HLM
human liver microsomes
Contributor Information
Charles W. Locuson, Department of Experimental and Clinical Pharmacology, College of Pharmacy, University of Minnesota, Minneapolis, MN.
Larry C. Wienkers, Department of Pharmacokinetics and Drug Metabolism, Amgen, Inc, Seattle, WA
Jeffrey P. Jones, Department of Chemistry and Program in Physical Biosciences, Washington State University, Pullman, WA
Timothy S. Tracy, Department of Experimental and Clinical Pharmacology, College of Pharmacy, University of Minnesota, Minneapolis, MN.
References
- Anthon GE, Barrett DM. Modified method for the determination of pyruvic acid with dinitrophenylhydrazine in the assessment of onion pungency. J Sci Food Agric. 2003;83:1210–1213. [Google Scholar]
- Backes WL, Kelley RW. Organization of multiple cytochrome P450s with NADPH-cytochrome P450 reductase in membranes. Pharmacol Ther. 2003;98:221–233. doi: 10.1016/s0163-7258(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Crespi CL, Miller VP. The use of heterologously expressed drug metabolizing enzymes--state of the art and prospects for the future. Pharmacol Ther. 1999;84:121–131. doi: 10.1016/s0163-7258(99)00028-5. [DOI] [PubMed] [Google Scholar]
- Gorsky LD, Coon MJ. Effects of conditions of reconstitution with cytochrome b5 on the formation of products in cytochrome P450-catalyzed reactions. Drug Metab Dispos. 1986;14:89–96. [PubMed] [Google Scholar]
- Gruenke LD, Konopka K, Cadieu M, Waskell L. The stoichiometry of the cytochrome P-450-catalyzed metabolism of methoxyflurane and benzphetamine in the presence and absence of cytochrome b5. J Biol Chem. 1995;270:24707–24718. doi: 10.1074/jbc.270.42.24707. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Role of cytochrome P450 enzymes in drug-drug interactions. Adv Pharmacol. 1997;43:7–35. doi: 10.1016/s1054-3589(08)60200-8. [DOI] [PubMed] [Google Scholar]
- Guryev OL, Gilep AA, Usanov SA, Estabrook RW. Interaction of apo-cytochrome b5 with cytochromes P4503A4 and P45017A: relevance of heme transfer reactions. Biochemistry. 2001;40:5018–5031. doi: 10.1021/bi002305w. [DOI] [PubMed] [Google Scholar]
- Haining RL, Jones JP, Henne KR, Fisher MB, Koop DR, Trager WF, Rettie AE. Enzymatic determinants of the substrate specificity of CYP2C9: Role of B ′-C loop residues in providing the pi-stacking anchor site for warfarin binding. Biochemistry. 1999;38:3285–3292. doi: 10.1021/bi982161+. [DOI] [PubMed] [Google Scholar]
- Hanna IH, Teiber JF, Kokones KL, Hollenberg PF. Role of the alanine at position 363 of cytochrome P450 2B2 in influencing the NADPH- and hydroperoxide-supported activities. Arch Biochem Biophys. 1998;350:324–332. doi: 10.1006/abbi.1997.0534. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Woollard AC, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- Jushchyshyn MI, Hutzler JM, Schrag ML, Wienkers LC. Catalytic turnover of pyrene by CYP3A4: evidence that cytochrome b5 directly induces positive cooperativity. Arch Biochem Biophys. 2005;438:21–28. doi: 10.1016/j.abb.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Kumar S, Davydov DR, Halpert JR. Role of cytochrome b5 in modulating peroxide-supported CYP3A4 activity: evidence for a conformational transition and cytochrome P450 heterogeneity. Drug Metab Dispos. 2005;33:1131–1136. doi: 10.1124/dmd.105.004606. [DOI] [PubMed] [Google Scholar]
- Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- Lang D, Bocker R. Highly sensitive and specific high-performance liquid chromatographic analysis of 7-hydroxywarfarin, a marker for human cytochrome P-4502C9 activity. J Chromatogr B Biomed Appl. 1995;672:305–309. doi: 10.1016/0378-4347(95)00222-5. [DOI] [PubMed] [Google Scholar]
- Locuson CW, Gannett PM, Tracy TS. Heteroactivator effects on the coupling and spin state equilibrium of CYP2C9. Arch Biochem Biophys. 2006;449:115–129. doi: 10.1016/j.abb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Coon MJ. Effects of cytochrome b5 on cytochrome P-450-catalyzed reactions. Studies with manganese-substituted cytochrome b5. Drug Metab Dispos. 1984;12:358–364. [PubMed] [Google Scholar]
- Nakajima M, Tane K, Nakamura S, Shimada N, Yamazaki H, Yokoi T. Evaluation of approach to predict the contribution of multiple cytochrome P450s in drug metabolism using relative activity factor: effects of the differences in expression levels of NADPH-cytochrome P450 reductase and cytochrome b(5) in the expression system and the differences in the marker activities. J Pharm Sci. 2002;91:952–963. doi: 10.1002/jps.10091. [DOI] [PubMed] [Google Scholar]
- Ollivon M, Lesieur S, Grabielle-Madelmont C, Paternostre M. Vesicle reconstitution from lipid-detergent mixed micelles. Biochim Biophys Acta. 2000;1508:34–50. doi: 10.1016/s0304-4157(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. Journal of Biological Chemistry. 1964;239:2370–2378. [PubMed] [Google Scholar]
- Perret A, Pompon D. Electron shuttle between membrane-bound cytochrome P450 3A4 and b(5) rules uncoupling mechanisms. Biochemistry. 1998;37:11412–11424. doi: 10.1021/bi980908q. [DOI] [PubMed] [Google Scholar]
- Porter TD. The roles of cytochrome b5 in cytochrome P450 reactions. J Biochem Mol Toxicol. 2002;16:311–316. doi: 10.1002/jbt.10052. [DOI] [PubMed] [Google Scholar]
- Rodrigues AD. Integrated cytochrome P450 reaction phenotyping - Attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol. 1999;57:465–480. doi: 10.1016/s0006-2952(98)00268-8. [DOI] [PubMed] [Google Scholar]
- Schenkman JB, Jansson I. The many roles of cytochrome b5. Pharmacol Ther. 2003;97:139–152. doi: 10.1016/s0163-7258(02)00327-3. [DOI] [PubMed] [Google Scholar]
- Tamburini PP, White RE, Schenkman JB. Chemical characterization of protein-protein interactions between cytochrome P-450 and cytochrome b5. J Biol Chem. 1985;260:4007–4015. [PubMed] [Google Scholar]
- Tracy TS, Hutzler JM, Haining RL, Rettie AE, Hummel MA, Dickmann LJ. Polymorphic variants (CYP2C9*3 and CYP2C9*5) and the F114L active site mutation of CYP2C9: effect on atypical kinetic metabolism profiles. Drug Metab Dispos. 2002;30:385–390. doi: 10.1124/dmd.30.4.385. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K, von Moltke LL, Court MH, Harmatz JS, Crespi CL, Greenblatt DJ. Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: ratios of accessory proteins as sources of discrepancies between the approaches. Drug Metab Dispos. 2000;28:1493–1504. [PubMed] [Google Scholar]
- Wrighton SA, Vandenbranden M, Stevens JC, Shipley LA, Ring BJ, Rettie AE, Cashman JR. In vitro methods for assessing human hepatic drug metabolism: their use in drug development. Drug Metab Rev. 1993;25:453–484. doi: 10.3109/03602539308993982. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Gillam EM, Dong MS, Johnson WW, Guengerich FP, Shimada T. Reconstitution of recombinant cytochrome P450 2C10(2C9) and comparison with cytochrome P450 3A4 and other forms: Effects of cytochrome P450-P450 and cytochrome P450-b(5) interactions. Arch Biochem Biophys. 1997;342:329–337. doi: 10.1006/abbi.1997.0125. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Johnson WW, Ueng YF, Shimada T, Guengerich FP. Lack of electron transfer from cytochrome b(5) in stimulation of catalytic activities of cytochrome P450 3A4-Characterization of a reconstituted cytochrome P450 3A4 NADPH-cytochrome P450 reductase system and studies with apo-cytochrome b(5) Journal of Biological Chemistry. 1996a;271:27438–27444. doi: 10.1074/jbc.271.44.27438. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, Yokoi T. Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr Purif. 2002;24:329–337. doi: 10.1006/prep.2001.1578. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakano M, Gillam EM, Bell LC, Guengerich FP, Shimada T. Requirements for cytochrome b(5) in the oxidation of 7-ethoxycoumarin, chlorzoxazone, aniline, and N-nitrosodimethylamine by recombinant cytochrome P450 2E1 and by human liver microsomes. Biochem Pharmacol. 1996b;52:301–309. doi: 10.1016/0006-2952(96)00208-0. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Shimada T, Martin MV, Guengerich FP. Stimulation of cytochrome P450 reactions by apo-cytochrome b5: evidence against transfer of heme from cytochrome P450 3A4 to apo-cytochrome b5 or heme oxygenase. J Biol Chem. 2001;276:30885–30891. doi: 10.1074/jbc.M105011200. [DOI] [PubMed] [Google Scholar]