Abstract
E2F3 and CDKAL1 are candidate genes from the 6p22 region frequently amplified in bladder cancer. Expression of E2F3 isoforms (E2F3a and b) and CDKAL1 were examined and modulated in 6p22-amplified bladder cell lines. Eight lines with amplification showed overexpression of both E2F3 isoforms and CDKAL1. shRNA-mediated knockdown of CDKAL1 had no effect on proliferation. Knockdown of E2F3a or E2F3b alone induced anti-proliferative effects, with the most significant effect on proliferation being observed when both isoforms were knocked down together. As E2Fs interact with the Rb tumour suppressor protein, Rb expression was analysed. There was a striking relationship between 6p22.3 amplification, E2F3 overexpression and lack of Rb expression. This was also examined in primary bladder tumours. Array-CGH detected 6p22.3 amplification in 8/91 invasive tumours. Five were studied in more detail. Four showed 13q14.2 loss (including RB1) and expressed no Rb protein. In the fifth, 13q was unaltered but the CDKN2A locus was deleted. This tumour was negative for p16 and positive for Rb protein. As p16 is a negative regulator of the Rb pathway, its loss represents an alternative mechanism for inactivation. Indeed, a phospho-specific Rb antibody showed much Rb protein in a hyperphosphorylated (inactive) form. We conclude that inactivation of the Rb pathway is required in addition to E2F3 overexpression in this subset of bladder tumours.
Keywords: E2F3, Rb, bladder cancer, 6p22 amplification
Introduction
Genomic alteration leading to gene amplification is a common mechanism for oncogene activation during cancer development. Conventional metaphase comparative genomic hybridisation (CGH) has detected recurrent gain and amplification at 6p22 in bladder tumours and cell lines (Bruch et al., 2000; Bruch et al., 1998; Kallioniemi et al., 1995; Prat et al., 2001; Tomovska et al., 2001) with a frequency of up to 30.8% (Aaboe et al., 2006). More recently, array-CGH has allowed higher resolution analysis of these genomic alterations (Blaveri et al., 2005; Hurst et al., 2004; Veltman et al., 2003). Previously, we detected high-level amplification at 6p22 in 4/22 bladder tumour-derived cell lines, allowing precise localisation of a minimum region of amplification spanning approximately 2.5Mb (Hurst et al., 2004), which corresponded well with critical regions delineated by others (Bruch et al., 2000; Feber et al., 2004; Veltman et al., 2003).
This region contains 4 genes (PRL, SOX4, E2F3 and CDKAL1). Other studies have identified DEK and ID4 as candidate oncogenes for 6p22 amplification (Evans et al., 2004; Wu et al., 2005) but these genes are telomeric to the critical region most commonly reported. PRL and SOX4 have been discounted as they are not in the peak region of 6p22 amplification (Feber et al., 2004) and their expression shows no clear relationship to amplification (Aaboe et al., 2006; Bruch et al., 2000; Hurst et al., 2004). Real-time RT-PCR analyses of CDKAL1 and E2F3 have revealed that overexpression of both genes correlates with 6p22 amplification in bladder tumour cell lines (Hurst et al., 2004; Olsson et al., 2007). Other studies also correlate overexpression of E2F3 with 6p22 amplification, higher bladder tumour stage and grade and higher proliferative index (Aaboe et al., 2006; Feber et al., 2004; Oeggerli et al., 2004).
CDKAL1 is named because of limited homology to the CDK5 regulatory subunit associated protein 1 but its function remains unknown. E2F3 belongs to the E2F family of transcription factors, of which eight family members (E2F1-8) and two associated dimerisation partner (DP) proteins have been identified (Frolov et al., 2001; Stevens & La Thangue, 2004; Trimarchi & Lees, 2002).
E2F3 encodes two protein products (E2F3a and E2F3b) through the use of alternative promoters and different 5′-coding exons (Leone et al., 2000). E2F3a and E2F3b share DNA binding, heterodimerisation and pocket protein binding domains and differ only in their N-termini (He et al., 2000; Leone et al., 2000). The functions of these 2 isoforms are not entirely clear but data suggests that they may have partially opposing roles. E2Fs have been subdivided into so-called “activators” and “repressors” based on their transcriptional regulation of target genes (reviewed in (Trimarchi & Lees, 2002)). E2F3a is classified as an activator E2F. It is expressed in proliferating cells, reaches peak levels in late G1 (Leone et al., 2000) and is linked to the transactivation of genes associated with DNA synthesis and cell cycle progression (Humbert et al., 2000). Activation of these targets is repressed during G1 when E2F3 is bound to Rb (Lees et al., 1993). During G1/S, Rb becomes hyperphosphorylated, leading to the release of E2F3a and target gene activation (reviewed in (Blais & Dynlacht, 2004; Dimova & Dyson, 2005; Trimarchi & Lees, 2002)). Overexpression of E2F3a stimulates quiescent cells to enter the cell cycle (DeGregori et al., 1997) and in mice in vivo, stimulates proliferation and p53-independent apoptosis (Paulson et al., 2006).
E2F3b has been classified as a repressor E2F, and is constitutively expressed throughout the cell cycle, including the quiescent phase (He et al., 2000; Leone et al., 2000). It is reported to be the predominant E2F3 partner for Rb in G0, implicating it in the process of controlling expression of targets specifically repressed by Rb in quiescent cells (He et al., 2000; Leone et al., 1998; Leone et al., 2000). Additionally, E2F3b has been implicated as a repressor of expression of the p19Arf tumour suppressor gene both in quiescent and normally proliferating cells (Aslanian et al., 2004).
Two recent studies have examined expression and function of 6p22 candidate genes in bladder cancer. Oeggerli et al. (Oeggerli et al., 2006) used bladder tumour tissue microarrays to examine the 6p22 amplification pattern and found that CDKAL1 and E2F3 were invariably co-amplified and co-overexpressed. Olsson et al. (Olsson et al., 2007) confirmed co-expression of CDKAL1 and E2F3 in amplified bladder tumour cell lines. Both studies showed that knockdown of expression of E2F3 but not of CDKAL1 resulted in reduced proliferation of 6p22-amplified cells, leading to the conclusion that E2F3 is the critical target within the amplicon. However, neither of these studies addressed the fact that the E2F3 locus encodes two isoforms (E2F3a and E2F3b), with potentially antagonistic functions.
As increased proliferative index is a key feature of bladder tumours with 6p22 amplification, we have focussed on the effects of modulation of expression of E2F3 and CDKAL1 on cell proliferation. We have also examined the expression of both E2F3 isoforms and have explored the individual role of these in functional assays. Our results show a functional link between E2F3a and E2F3b overexpression and proliferative advantage in tumour cells with 6p22 amplification. As Rb is involved in the regulation of E2F3-induced proliferation and in light of the fact that Feber et al. (Feber et al., 2004) reported loss of Rb function in three 6p22-amplified bladder cell lines, we have examined a large series of primary tumours for 6p22 amplification and loss of Rb. Our findings point to an obligate interaction of these alterations, with both inactivation of the Rb pathway and overexpression of E2F3a and E2F3b being necessary events in tumours with 6p22 amplification.
Results
Amplicon mapping and expression analysis of candidate genes confirm E2F3 and CDKAL1 as candidate bladder oncogenes
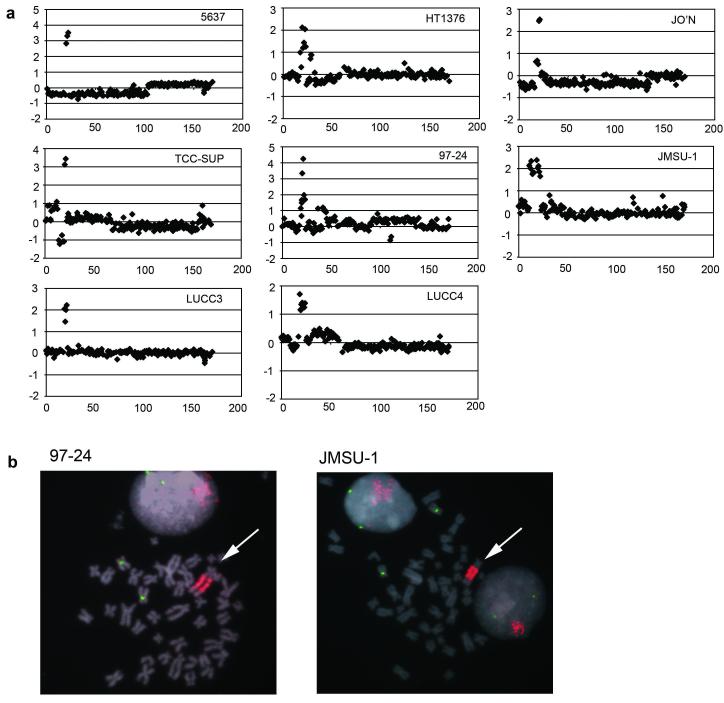
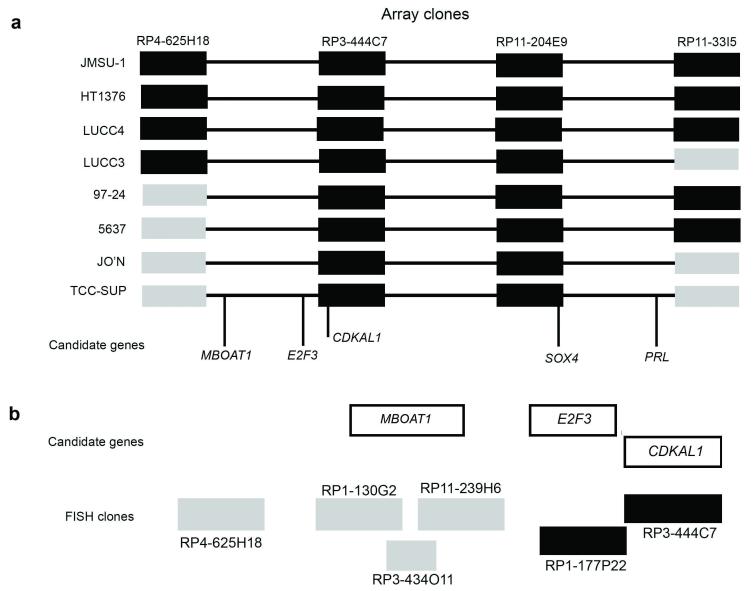
Previously, we identified 6p22 amplification in 4 of 22 bladder cell lines (Hurst et al., 2004). Additional array-CGH studies revealed amplification in four more cell lines (97-24, JMSU-1, LUCC3 and LUCC4). Array-CGH plots for all 8 cell lines and FISH confirmation of amplification in 97-24 and JMSU-1 are shown in Figure 1. We defined a minimum region of amplification at 6p22 based on data for cell lines 5637, HT1376, JO’N and TCC-SUP (Hurst et al., 2004). The amplicons identified in 97-24, JMSU-1, LUCC3 and LUCC4 included this minimum region but did not refine it (Figure 2a). Candidate genes within the minimum region are PRL, SOX4, E2F3 and CDKAL1. Another gene, MBOAT1, was recently mapped to this region distal to E2F3. To assess MBOAT1 as a potential candidate oncogene, we performed FISH analysis on metaphase spreads of TCC-SUP using BAC clones covering the MBOAT1 gene locus as probes. None of these BAC clone probes (RP1-130G2; RP11-239H6; RP3-434O11) revealed the presence of amplification (Figure 2b).
Figure 1.
6p22 amplification and FISH confirmation in bladder tumour-derived cell lines. (a) array-CGH detection of 6p22 amplification in bladder tumour-derived cell lines 5637, HT1376, JO’N, TCC-SUP, 97-24, JMSU-1, LUCC3 and LUCC4. Results are presented as individual chromosome 6 plots of log2 ratio versus distance along chromosome (Mb). (b) FISH confirmation of 6p22 amplification in cell lines 97-24 and JMSU-1. BAC clone RP11-204E9 (red) was used as a probe for the amplified region together with a chromosome 6 centromere probe (green). Arrows indicate amplified material.
Figure 2.
Minimal region of amplification at 6p22 and candidate genes. Schematic representations illustrating (a) amplified and non-amplified clones in 8 bladder tumour cell lines with 6p22 amplification and candidate genes within a minimal region and (b) refinement of the minimal region distal to E2F3 using FISH. Amplified and non-amplified clones are represented by black and grey boxes, respectively.
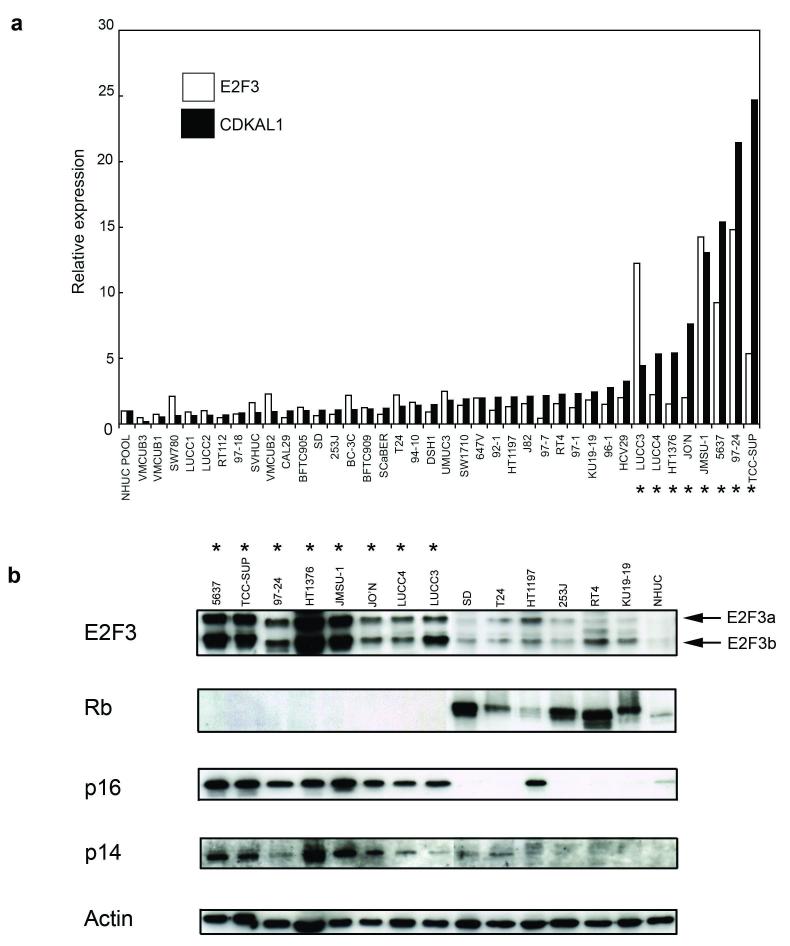
We showed previously by real-time RT-PCR that PRL was not expressed and SOX4 expression was not related to 6p22 amplification (Hurst et al., 2004). However, a relationship between elevated expression of E2F3 and CDKAL1 and the presence of the amplicon was observed in the 4 cell lines examined, with a closer correlation between CDKAL1 expression and the presence of amplification. We have now examined a panel of 39 bladder cell lines, including 97-24, JMSU-1, LUCC3 and LUCC4 for expression of E2F3 and CDKAL1 mRNA by real-time RT-PCR. A similar relationship was observed for these cell lines, with CDKAL1 mRNA expression again showing the closest relationship to amplification (Figure 3a).
Figure 3.
Real time RT-PCR analysis and western blot analysis of bladder tumour-derived cell lines with (*) and without 6p22 amplification. (a) the relative expression of candidate genes CDKAL1 (black bars) and E2F3 (white bars) was determined by real-time RT-PCR. Expression levels were normalised to the control gene SDHA and relative to a pooled normal human urothelial cell (NHUC) sample. (b) ten micrograms of protein extract from a sub-panel of cell lines were analysed by western blotting with antibodies specific to E2F3, Rb, p16 or p14. The E2F3 antibody was specific for the c-terminus of the protein and detected E2F3a and b isoforms as indicated.
E2F3a and E2F3b isoforms are both overexpressed at the protein level in 6p22-amplified cell lines
Expression of E2F3 was analysed by western blotting analysis using an antibody recognising the c-terminus of the protein. Two isoforms of E2F3 (E2F3a and E2F3b) were detected and both were over-expressed in 6p22-amplified cell lines relative to NHUC and cell lines lacking 6p22 amplification (Figure 3b). Levels of E2F3 protein showed a much closer relationship to amplification status than mRNA levels. We were unable to analyse expression of CDKAL1 protein as there are no antibodies currently available.
siRNA-mediated knockdown of both E2F3 isoforms results in reduced proliferation
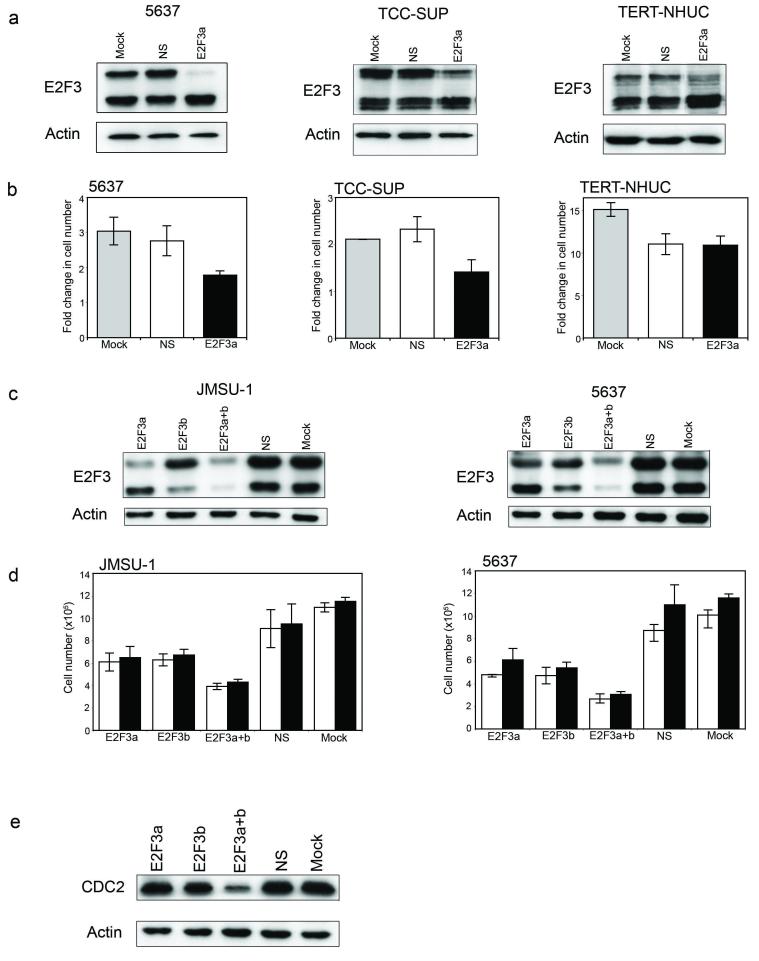
Two recent studies performed siRNA knockdown of E2F3 in bladder cell lines with 6p22 amplification and showed that knockdown led to a reduced rate of proliferation (Oeggerli et al., 2006; Olsson et al., 2007). Olsson et al. (Olsson et al., 2007) employed siRNA probes targeting both E2F3 isoforms simultaneously and Oeggerli et al. (Oeggerli et al., 2006) did not specify the position of their siRNA probes or the isoforms they were directed against. To address this issue, we initially designed an siRNA oligonucleotide to specifically target E2F3a. Cell lines 5637 and TCC-SUP were treated with transfection reagent only or transfected with non-specific control (NS) or E2F3a-specific siRNA, and cells harvested for protein analysis or counts 3 or 4 days later. Western blot analysis confirmed specific knockdown of E2F3a (Figure 4a). Adherent cell counts showed that the fold-change in cell number was significantly lower in E2F3a-knockdown cells compared to controls (Figure 4b). Interestingly, specific knockdown of E2F3a was accompanied by a corresponding increase in the amount of E2F3b protein (Figure 4a). The same experiment was performed in NHUC-TERT cells and no such effect on proliferation was observed (Figures 4a and 4b). These normal cells showed increased sensitivity to oligonuclotide transfection compared to tumour cells. Thus although there was no significant difference in response to non-specific or specific siRNAs, transfected cells showed consistent inhibition compared with mock-transfected controls (Figure 4b).
Figure 4.
Oligonucleotide-mediated siRNA knockdown of E2F3 in bladder tumour-derived cell lines with 6p22 amplification and telomerase-immortalised normal human urothelial cells (NHUC-TERT). (a) Western blot analysis of E2F3 in protein lysates from 6p22-amplified cells (5637 and TCC-SUP) and NHUC-TERT targeted with E2F3a-specific siRNA oligonucleotides. (b) Fold change in adherent cell number of 5637, TCC-SUP and NHUC-TERT cells with targeted knockdown of E2F3a expression. (c) Western blot analysis of E2F3 in protein lysates from 6p22-amplified cells (JMSU-1 and 5637) targeted with E2F3a-, E2F3b- or E2F3a+b-specific siRNA oligonucleotides. (d) Viable (white bars) and total (black bars) cell counts of JMSU-1 and 5637 cells targeted with E2F3 siRNA oligonucleotides. (e) Western blot analysis of CDC2 expression in JMSU-1 cells with targeted knockdown of E2F3.
Subsequently, we designed two additional siRNA oligonucleotides targeting either E2F3b alone or both isoforms (siRNA E2F3a+b) and used these and the E2F3a-specific siRNA in knockdown experiments with JMSU-1 and 5637 cells. Knockdown of E2F3a, E2F3b and E2F3a+b was confirmed by western blotting (Figure 4c). Viable and total cell numbers were significantly lower in cells transfected with E2F3a-specific, E2F3b-specific or E2F3a+b-specific siRNAs compared to controls, with the most significance difference when both E2F3 isoforms were targeted together (Figure 4d). No significant difference in the proportion of viable to total cells nor in the percentage of apoptotic cells was observed between siRNA treatments and controls, (data not shown). siRNA-treated NHUC-TERT cells showed no significant difference in viable and total cell counts (data not shown).
To determine whether the reduction in cell numbers was due to reduction in the proportion of cells in S-phase, we measured BrdU incorporation on replicate plates from the JMSU-1 knockdown experiment described above. The proportion of siRNA-treated cells in S-phase was significantly lower (E2F3a, 67%; E2f3b, 65%; E2F3a+b, 48%) compared to controls (NS, 72%; Mock, 71%), with the most significant difference when both forms of E2F3 were knocked down together. To see if this reduction in S-phase fraction correlated with expression of an E2F3 target involved in G1/S transition, we examined CDC2 which was previously identified as downregulated at the mRNA level in 5637 E2F3-knockdown cells (Olsson et al., 2007). A reduction in the level of CDC2 protein in JMSU1 E2F3-knockdown cells was found that reflected the proportion of cells in S-phase. The greatest reduction in the level of CDC2 protein was in cells transfected with the E2F3a+b siRNA (Figure 4e).
Retroviral-mediated siRNA modulation of CDKAL1 expression has no effect on cell proliferation
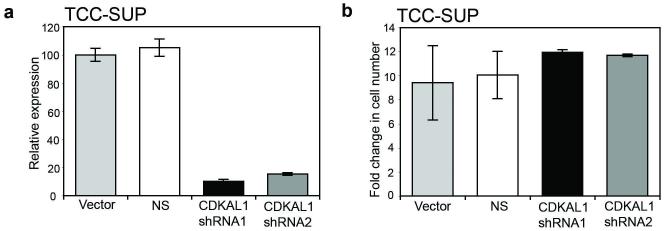
Oeggerli et al. (Oeggerli et al., 2006) and Olsson et al. (Olsson et al., 2007) used oligonucleotide approaches to siRNA targeting of CDKAL1 expression in 6p22-amplified bladder cell lines and showed that knockdown had no effect on cell proliferation rate. To confirm these results in cells with stable siRNA knockdown, we transduced TCC-SUP cells with retroviruses containing shRNAs targeting CDKAL1. Knockdown was confirmed by real-time RT-PCR (Figure 5a). Stable transductants were plated and cell counts made on day 5. No significant difference in cell number between cells expressing CDKAL1 shRNAs and cells transduced with vector only or non-specific controls was observed (Figure 5b). These results indicate that CDKAL1 overexpression does not confer a proliferative advantage in 6p22-amplified bladder tumour cells.
Figure 5.
Retroviral-mediated knockdown of CDKAL1 in 6p22-amplified cell line TCC-SUP. (a) Real-time RT-PCR was used to confirm knockdown of CDKAL1. Levels of expression were normalised to the SDHA control gene and relative to a vector control. (b) Fold change in adherent cell number of cells stably expressing CDKAL1 shRNAs.
Tumour cell lines with 6p22.3 amplification all show loss of Rb expression
Rb is a key regulator of E2F3, and Feber et al. (Feber et al., 2004) reported that three bladder cell lines with 6p22 amplification (5637, TCC-SUP and HT1376) lacked functional Rb. Thus we were interested to examine Rb status in a larger panel of cell lines with 6p22 amplification. We found a striking relationship between Rb expression and presence of the 6p22 amplicon, with all 8 amplified cell lines lacking any detectable Rb protein (Figure 3b).
Inactivation of p16 is an alternative mechanism to deregulate the Rb pathway. This is common in bladder cancer and is most often achieved via homozygous deletion of the entire CDKN2A locus on 9p21, which eliminates the coding region of both p16 and p14ARF (Chapman et al., 2005). Five of the 6p22-amplified cell lines (5637, HT1376, JO’N, 97-24, TCC-SUP) are known to retain this locus at the genomic level (data not shown). All 8 of the amplified cell lines showed moderate to high levels of expression of both p16 and p14ARF (Figure 3B).
Inactivation of the Rb pathway occurs in bladder tumours with 6p22 amplification
To date the relationship between 6p22 amplification and Rb expression has not been examined in primary tumour samples. To exclude the possibility that the relationship between E2F3 expression, Rb status and the presence of the 6p22 amplicon represents either an artefact of tissue culture of bladder tumour cells or the selection in vitro of a minor sub-set of tumours, we sought to confirm our findings in primary tumour tissues. We focussed on invasive tumours as these have been shown by others to have a higher frequency of 6p22 amplification and E2F3 overexpression. Ninety-one muscle-invasive (≥pT1) tumours were analysed by array-CGH and amplification of 6p22 was detected in 8 of these (9%).
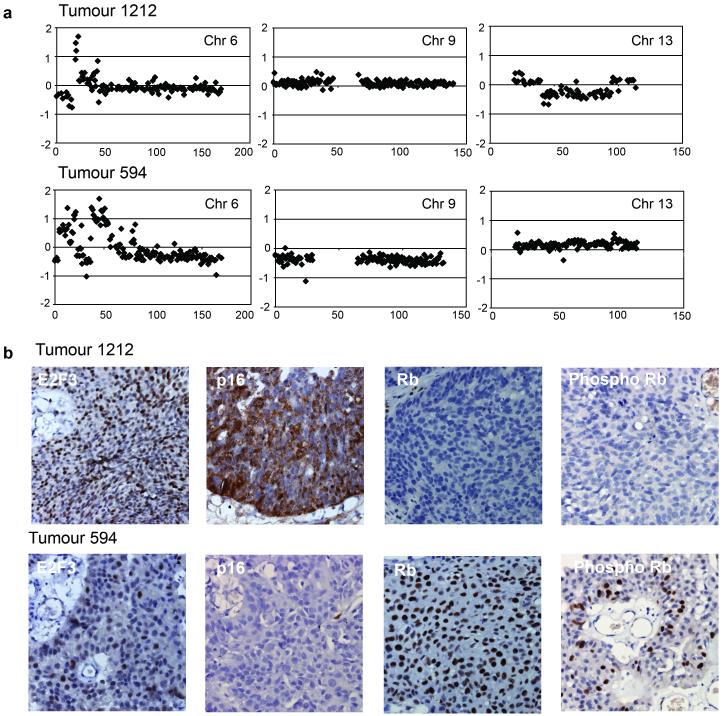
Five of these tumours were studied in more detail. Four showed copy number loss at 13q14.2 (including the RB1 gene) and a lack of Rb protein expression by immunohistochemistry. Representative results for one of these (tumour 1212) are shown in Figure 6. In the fifth tumour (tumour 594), the RB1 gene region was unaltered but copy number loss at 9p21.3, encompassing the CDKN2A locus (encoding p16 and p14ARF), was observed (Figure 6a). Immunohistochemical staining of this tumour showed a lack of p16 expression and positive staining for Rb (Figure 6b). As p16 is a negative regulator of the Rb pathway, this may provide an alternative mechanism for Rb inactivation in this case. A phospho-specific Rb antibody showed that a significant proportion of the protein present was in a hyperphosphorylated, inactivated form (Figure 6b).
Figure 6.
Array-CGH and immunohistochemical analysis of bladder tumours with 6p22 amplification. (a) Array-CGH analysis of bladder tumours 1212 and 594. Individual chromosome plots of log2 ratio versus distance along chromosome (Mb) are shown for chromosomes 6, 9 and 13. (b) Immunohistochemical analysis of E2F3, p16, Rb and phospho-Rb in bladder tumours 1212 and 594.
Discussion
An association between 6p22 amplification in bladder cancer and overexpression of two candidate genes (E2F3 and CDKAL1) has been reported previously (Hurst et al., 2004; Oeggerli et al., 2006; Olsson et al., 2007) and we have confirmed this in a larger sample panel in this study. We have also addressed the functional significance of 6p22 amplification via siRNA mediated knockdown experiments, have dissected the individual roles of E2F3a and E2F3b isoforms and have demonstrated the relationship between Rb inactivation and E2F3 amplification in primary tumour samples.
Recent studies using siRNA knockdown of CDKAL1 in 6p22 amplified bladder cell lines demonstrated that knockdown had no effect on proliferation rate (Oeggerli et al., 2006; Olsson et al., 2007) and we have confirmed this in an additional amplified cell line TCC-SUP. Possibly it is coincidental that CDKAL1 is co-amplified with E2F3 because of its proximity, or perhaps it exerts its oncogenic properties in a way that is not related to proliferation. Interestingly, genome-wide association studies have recently identified association of intronic variants of CDKAL1 with risk of type 2 diabetes and reduced insulin response (Saxena et al., 2007; Scott et al., 2007; Steinthorsdottir et al., 2007; Zeggini et al., 2007). The level of CDKAL1 mRNA is consistently high in amplified samples and further studies are now required to elucidate its potential oncogenic role, possibly in relation to glucose metabolism.
The evidence that E2F3 is a key target within this amplicon is now overwhelming. Previous studies did not address the role of the two E2F3 isoforms which have potentially different functions and we have now shown that both isoforms of E2F3 are overexpressed in 6p22-amplified bladder tumour cell lines and that siRNA-mediated knockdown of either isoform results in reduced proliferation, with the most significant effect when dual knockdown is employed. The effects on proliferation are compatible with E2F3a’s role in transactivation of genes involved in cell cycle progression (Dyson, 1998). Indeed, we found that a downstream target of E2F3, CDC2, which is involved in G1/S transition was downregulated in E2F3 knockdown cells. Upregulation of E2F3a expression appears to provide tumour cells with a proliferative advantage in vitro, compatible with the association of E2F3 amplification with higher proliferative index in bladder tumours in vivo (Oeggerli et al., 2004; Tomovska et al., 2001). Expression of E2F3 in quiescent fibroblasts induces progression into S phase (DeGregori et al., 1997). Conversely, microinjection of E2F3 antibodies blocks S phase entry (Leone et al., 1998) and cultured E2F3-/- MEFs exhibit proliferation defects (Humbert et al., 2000). In transgenic mice, ectopic expression of E2F3a in squamous epithelia led to hyperplasia, though overexpression in the urinary bladder did not affect proliferation (Paulson et al., 2006). Possibly, response to deregulated E2F3a may be tissue-specific and other events may be required in urothelial cells to permit E2F3-mediated cell cycle dysregulation.
E2F3b is predicted to act as a repressor E2F. p19ARF is a known target of this repression and it has been suggested that E2F3b represses p19ARF during periods where no oncogenic stress is present. E2F3b/Rb is the predominant E2F/Rb complex in some quiescent cells (Leone et al., 2000) implicating it in control of genes that are specifically repressed by Rb in G0. Thus, the observation that E2F3b knockdown in 6p22-amplified bladder cell lines reduces proliferation was unexpected and suggests that in these cells E2F3b and E2F3a co-operate to positively affect proliferation. An interesting observation was the inverse relationship between levels of E2F3a and E2F3b proteins in knockdown experiments, indicating that there may be regulatory feedback. No other studies have addressed the expression levels of the two isoforms or dissected their roles in human cancers. Future studies must address the precise function of E2F3b in tumour cells.
We showed that knockdown of E2F3 in NHUC-TERT cells has no effect on proliferation, implying tight regulation of E2F3 in normal urothelium. Previous siRNA experiments in a non-amplified bladder tumour-derived cell line (CRL-7930), found that E2F3 knockdown reduced proliferation rate (Oeggerli et al., 2006). As E2F3 expression is regulated in part by p16, and p16 loss is common in bladder tumours, this could be related to p16 status. We have measured E2F3, p16 and Rb protein expression in a large panel of non-amplified bladder tumour-derived cell lines (data not shown). Many are p16 null, exhibit high levels of phosphorylated (inactive) Rb and express elevated levels of E2F3 compared to NHUC. Though levels are much lower than in 6p22-amplified cells, there may nevertheless be some E2F3-dependence for proliferation.
Others have reported that 6p22-amplified bladder tumour-derived cell lines (TCC-SUP, HT1376 and 5637) lack functional Rb (Feber et al., 2004). We confirmed this relationship in five more bladder cell lines with amplification. This reinforces the suggestion that Rb pathway inactivation is an obligate event in bladder tumour cells with E2F3 over-expression. A co-operative relationship between E2F3 overexpression and Rb inactivation was also reported for retinoblastoma (Orlic et al., 2006) and small cell lung cancer (Cooper et al., 2006). Several studies have shown inactivation of Rb in bladder cancer (Cordon-Cardo et al., 1992; Logothetis et al., 1992; Xu et al., 1993) and lack of expression has been associated with tumour progression (Cote et al., 1998). A comprehensive study of the relationship of E2F3 expression and Rb inactivation has not yet been carried out.
There is complex interplay between E2F1, E2F3 and Rb in controlling the balance between proliferation and apoptosis in response to inappropriate proliferative stimuli and during tumour development in mice. In mouse models, Rb loss results in 100% incidence of pituitary tumours but although over-expression of E2F3 induces proliferation and hyperplasia, this is not sufficient to induce tumour formation (Lazzerini Denchi et al., 2005). However, loss of E2F3 decreases pituitary tumour incidence in Rb+/- mice, indicating a positive contribution of E2F3 to tumour development (Ziebold et al., 2003). As E2F3 over-expression leads to increased E2F1-dependent apoptosis both in these transgenic mice and in cultured MEFs (Lazzerini Denchi et al., 2005), this is compatible with the finding that in Rb-/-MEFs, the inappropriate proliferation and apoptosis observed can be rescued by deletion of E2F3 (Ziebold et al., 2001). Thus, the anti-apoptotic effect of E2F3 removal in Rb null cells appears to be via loss of an E2F1 effect. Thus, abrogation of the pro-apoptotic function of E2F1 may be required for loss of Rb and over-expression of E2F3 to act in concert. We hypothesise that in tumour cells with p53 pathway inactivation, this E2F1 function may be sufficiently attenuated to be permissive for enhanced proliferation in the presence of both loss of Rb and over-expression of E2F3a. In support of this, the TP53 mutation status of 5 of the cell lines with 6p amplification studied here is known and all are mutant (data not shown). Our finding of increased p14ARF levels in all cell lines with amplification is compatible with p53 loss and/or inappropriate proliferative signalling (Stott et al., 1998). Indeed 6 of the 8 lines show stabilised p53 protein levels. Alternatively high levels of p14ARF may reflect loss of an Rb/E2F3b effect on transcription. The relationship between Rb and E2F3 expression has been examined by expression of E2F3a in wild-type (PC3) or Rb null (DU145) prostate cancer cell lines. Ectopic expression of E2F3a enhanced proliferation rate in DU145 cells but had no effect on PC3 cells unless Rb levels were reduced by siRNA knockdown (Olsson et al., 2007). Both of these cell lines contain TP53 mutations.
All 6p22-amplified bladder cell lines examined here showed moderate to high levels of p16 expression. This inverse relationship between Rb and p16 expression has been described previously (Benedict et al., 1999; Le Frere-Belda et al., 2004; Yeager et al., 1995) and has been suggested to represent a feedback effect in the presence of a disrupted Rb pathway (Chatterjee et al., 2004). Array-CGH analysis of primary bladder tumours with 6p22 amplification showed copy number loss at 13q14.2 (including the RB1 gene locus) in four of five tumours examined. Immunohistochemistry revealed a lack of Rb protein expression and strong p16 protein staining in these tumours, reflecting the situation seen in 6p22-amplified bladder cell lines. In the other tumour, copy number at the RB1 gene locus was unaltered but loss at 9p21.3 encompassing the CDKN2A locus (encoding p16 and p14) was observed. Immunohistochemistry revealed lack of p16 protein expression and positive staining for Rb. In the absence of p16, the cdk4-cdk6/Cyclin D enzyme complex would be free to phosphorylate Rb, resulting in the release of E2F3 and subsequent cell cycle progression. Hyperphosphorylation of Rb by the cdk4-cdk6/Cyclin D complex has been reported and proposed as an alternative mechanism for inactivation of Rb in bladder tumours lacking p16 (Chatterjee et al., 2004). Here, a phospho-specific Rb antibody did indeed reveal that a significant proportion of the protein in the tumour lacking p16 was in a hyperphosphorylated, inactivated form. The relationship between elevated E2F3 expression, p16 and Rb/phospho Rb status in amplified and non-amplified bladder tumours clearly merits further investigation.
In conclusion, two alternative mechanisms exist in bladder tumours with 6p22 amplification, both of which result in inactivation of Rb. We propose that inactivation of the Rb pathway and overexpression of both forms of E2F3 are obligate events in bladder tumour cells with 6p22 amplification.
Materials and Methods
Cell lines and culture conditions
Thirty-seven bladder tumour-derived cell lines (5637, 253J, 647V, 92-1, 94-10, 96-1, 97-1, 97-18, 97-24, 97-7, BC-3C, BFTC905, BFTC909, CAL29, DSH1, HT1197, HT1376, J82, JMSU-1, JO’N, KU19-19, LUCC1, LUCC2, LUCC3, LUCC4, RT112M, RT4, SCaBER, SD, SW1710, SW780, T24, TCC-SUP, UMUC3, VMCUB1, VMCUB2, VMCUB3), one SV40 large T antigen immortalised urothelial cell line (SVHUC) (Christian et al., 1987) and one cell line derived from non-malignant urothelium of a TCC patient treated with radiotherapy (HCV29) (Paulie, 1983) were used. Cell lines were cultured in standard growth media at 37°C in 5% CO2. Primary normal human urothelial cells (NHUC) or telomerase immortalised NHUC (TERT-NHUC) were maintained as described (Chapman et al., 2006).
DNA extraction, array-CGH and fluorescence in situ hybridisation (FISH)
DNA was extracted using a QIAmp DNA mini kit (Qiagen, Crawley, UK). 1Mb resolution CGH arrays were used for genome-wide analysis of DNA copy number (array details available upon request). Hybridisation to arrays was essentially as previously described (Fiegler et al., 2003; Hurst et al., 2004). DNA from BAC/PAC clones selected for FISH confirmation of array-CGH results and fine mapping of the 6p22 amplicon was prepared as described (www.sanger.ac.uk/HGP/methods/cytogenetics). 100ng of DNA was labelled with either biotin-14-dATP or digoxigenin-11-dUTP using a BioPrime DNA Labelling Kit (Invitrogen). FISH was performed as described previously (Hurst et al., 2004).
RNA extraction, cDNA synthesis and real time RT-PCR
Total RNA was extracted using an RNeasy mini kit (Qiagen), and cDNA was synthesised using Superscript™ II (Invitrogen) according to the manufacturer’s instructions. Real time RT-PCR was performed as described previously (Hurst et al., 2004) except that control gene primers were designed to succinate dehydrogenase subunit A (SDHA) (forward 5′-TGGGAACAAGAGGGCATCTG-3′, reverse 5′-CCACCACTGCATCAAATTCATG). Levels of expression were relative to a pooled NHUC sample in cell line expression studies, and to “mock” or “vector” control samples in knockdown studies.
Protein isolation and western blot analysis
Cells were lysed in RIPAE buffer (1% Triton X-100, 1mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS in PBS) containing protease inhibitors (P8340; Sigma) and phosphatase inhibitors (P5726; Sigma) and cleared by centrifugation. 20μg of protein were loaded onto 8-15% SDS polyacrylamide gels then transferred to Hybond™-C super membrane (Amersham Biosciences, Little Chalfont, UK). Primary antibodies specific to p14ARF (1:1000; 4C6; Cancer Research UK, London, UK), p16 (1:500; C-20; Santa Cruz Biotechnology, CA, USA), Rb (1:500; G3-245; BD Pharmingen, San Diego, USA), CDC2 (1:200; Santa Cruz Biotechnology) and the c-terminal portion of E2F-3 (1:200; C-18; Santa Cruz Biotechnology) were used. Horseradish peroxidase-conjugated secondary antibodies and an ECL Plus chemiluminescence kit (Amersham Biosciences) were used to detect bound antibody. Blots were incubated in stripping buffer (50mM Tris pH 7.5, 10M urea) for 1 hr at 55°C, and re-probed with an actin antibody (1:1000; AC-40; Sigma) as loading control.
Immunohistochemistry
3μm sections of formalin-fixed, paraffin wax-embedded bladder tumours were deparaffinised in xylene, rehydrated through graded ethanols and endogenous peroxidase activity blocked in 3% hydrogen peroxide. For antigen retrieval, sections were boiled in 10mM citric acid buffer (pH6) (Rb; phospho-Rb) or 1mM EDTA (pH8) (E2F3) for 10 min and non-specific binding was blocked by incubation with avidin/biotin solution (Vector Laboratories, Burlingame, CA, USA) followed by 10% normal serum (Dako Cytomation, Glostrup, Denmark). No antigen retrieval was performed for p16. Slides were incubated with primary antibodies to E2F3 (1:200; Clone 3E2F04; Lab Vision), p16 (1:1500; 16P07; Lab Vision), Rb (1:4000; Clone 3C8; Autogen Bioclear), or phospho-Rb (1:100; Ser807/811; Cell Signaling Technology Inc., Danvers, MA, USA) for 60 minutes at room temperature followed by species-specific secondary antibody for 30 minutes. Incubation with StreptAB-Complex (Dako Cytomation) for 30 minutes was followed by visualisation with 3,3′diamino-benzidine terahydrochloride (DAB; Vector Laboratories) and counterstaining with haematoxylin. All runs included a no primary antibody control. The specificity of phospho-Rb antibody was confirmed by phosphatase treatment of sample sections using Lambda protein phosphatase (λ-PPase; New England Biolabs, Hertfordshire,UK) prior to staining.
Oligonucleotide-mediated siRNA knockdown
Sense and antisense oligonuleotides were designed to target E2F3a (sense 5′-GCGUACAUCCAGAUCCUCAUU-3′, antisense 5′-UGAGGAUCUGGAUGUACGCUU-3′), E2F3b (sense 5′-GGAAAUGCCCUUACAGCAGUU-3′, antisense 5′-CUGCUGUAAGGGCAUUUCCUU-3′) and E2F3 a+b (sense 5′-GACCAAACUGUUAUAGUUGUU-3′, antisense 5′-CAACUAUAACAGUUUGGUCUU-3′). Annealed oligonucleotides and a non-specific control siRNA (siCONTROL Non-targeting siRNA # 1) were obtained from Dharmacon (Chicaco, USA). Optimum plating densities for transfection were determined for each cell line. Cells were plated in replicate 6 well dishes allowing repeated sampling of cell number, RNA and protein. Transfections were performed using 100nM of siRNA and 4μl of DharmaFECT Transfection Reagent (Dharamcon) according to the manufacturer’s instructions.
shRNA constructs, production of retroviruses and transductions
Forward and reverse oligonucleotides were designed to target CDKAL1 (shRNA1 forward 5′-GCTCAAGAGGAGAACAAGATTCAAGAGATCTTGTTCTCCTCTTGAGCTTTTTTGGGCC-3′, shRNA1 reverse 5′-CAAAAAAGCTCAAGAGGAGAACAAGATCTCTTGAATCTTGTTCTCCTCTTGAGC-3′; shRNA2 forward 5′-GAATCCACTGATAGAAATCTTCAAGAGAGATTTCTATCAGTGGATTCTTTTTTGGGCC-3′, reverse 5′-CAAAAAAGAATCCACTGATAGAAATCTCTCTTGAAGATTTCTATCAGTGGATTC-3′) or a non-specific (NS) target (forward 5′-CTTCAGCCGTTACGCTCGGTTCAAGAGACCGAGCGTAACGGCTGAAGTTTTTGGGCC-3′, reverse 5′-CAAAAACTTCAGCCGTTACGCTCGGTCTCTTGAACCGAGCGTAACGGCTGAAG-3′). Cloning of shRNAs into pRetroSuper-puro (pRS-puro), retrovirus production and transductions were performed as previously described (Tomlinson et al., 2007).
Adherent and viable cell counts
Cells were plated at equal densities. Initial counts and a subsequent count 3 to 6 days later were performed from triplicate wells at each time point. Adherent cells were counted using a Z2 Coulter Particle Count and Size Analyzer (Beckman Coulter UK Ltd, High Wycombe, UK) and fold-change was calculated by comparing initial to subsequent counts. Viable cells were stained using the Guava PCA-96 ViaCount Flex Reagent (Guava Technologies Inc, Hayward, CA) according to the manufacturer’s instructions. Viable counts were determined by flow cytometry using a Guava EasyCyte Plus System with Cytosoft™ software (Guava Technologies Inc.).
Apoptosis and proliferation assays
Apoptotic cells were stained using a Guava-96 Nexin™ Kit (Guava Technologies) according to the manufacturer’s instructions. Percentages of apoptotic cells were determined by flow cytometry. Bromodeoxyuridine (BrdU) and propidium iodide (PI) staining was used to analyse the percentage of proliferative cells. Cells were incubated for 1 hr at 37°C in culture medium containing BrdU at a final concentration of 10μM, then trypsinised, fixed in 70% ethanol and left overnight at 4°C. Fixed cells were re-hydrated in PBS then treated with 2M HCl for 20 min at room temperature. After two washes in PBS, cells were incubated with a mouse monoclonal anti-BrdU antibody (1:100; Dako) for 1hr at room temperature, then washed twice in PBS and incubated for 1 hr at room temperature with an anti-mouse immunoglobulins FITC-conjugated secondary antibody (1:50; Dako). This was followed by further washes in PBS and staining with propidium iodide. Stained cells were analysed by flow cytometry.
Acknowledgements
This work was supported by a programme grant from Cancer Research UK (C6228/A5433). The authors would like to thank Jo Brown and Filomena Esteves for their help with tissue collection and immunohistochemistry. U6 promoter and pRetroSuper constructs were kind gifts from Dr. D. Takai and Dr. R. Agami, respectively.
References
- Aaboe M, Birkenkamp-Demtroder K, Wiuf C, Sorensen FB, Thykjaer T, Sauter G, et al. SOX4 expression in bladder carcinoma: clinical aspects and in vitro functional characterization. Cancer Res. 2006;66:3434–3442. doi: 10.1158/0008-5472.CAN-05-3456. [DOI] [PubMed] [Google Scholar]
- Aslanian A, Iaquinta PJ, Verona R, Lees JA. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 2004;18:1413–1422. doi: 10.1101/gad.1196704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict WF, Lerner SP, Zhou J, Shen X, Tokunaga H, Czerniak B. Level of retinoblastoma protein expression correlates with p16 (MTS-1/INK4A/CDKN2) status in bladder cancer. Oncogene. 1999;18:1197–1203. doi: 10.1038/sj.onc.1202452. [DOI] [PubMed] [Google Scholar]
- Blais A, Dynlacht BD. Hitting their targets: an emerging picture of E2F and cell cycle control. Curr Opin Genet Dev. 2004;14:527–532. doi: 10.1016/j.gde.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Blaveri E, Brewer JL, Roydasgupta R, Fridlyand J, DeVries S, Koppie T, et al. Bladder cancer stage and outcome by array-based comparative genomic hybridization. Clin Cancer Res. 2005;11:7012–7022. doi: 10.1158/1078-0432.CCR-05-0177. [DOI] [PubMed] [Google Scholar]
- Bruch J, Schulz WA, Haussler J, Melzner I, Bruderlein S, Moller P, et al. Delineation of the 6p22 amplification unit in urinary bladder carcinoma cell lines. Cancer Res. 2000;60:4526–4530. [PubMed] [Google Scholar]
- Bruch J, Wohr G, Hautmann R, Mattfeldt T, Bruderlein S, Moller P, et al. Chromosomal changes during progression of transitional cell carcinoma of the bladder and delineation of the amplified interval on chromosome arm 8q. Genes Chromosomes Cancer. 1998;23:167–174. doi: 10.1002/(sici)1098-2264(199810)23:2<167::aid-gcc10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Harnden P, Chambers P, Johnston C, Knowles MA. Comprehensive analysis of CDKN2A status in microdissected urothelial cell carcinoma reveals potential haploinsufficiency, a high frequency of homozygous co-deletion and associations with clinical phenotype. Clin Cancer Res. 2005;11:5740–5747. doi: 10.1158/1078-0432.CCR-05-0411. [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Hurst CD, Pitt E, Chambers P, Aveyard JS, Knowles MA. Expression of hTERT immortalises normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene. 2006;25:5037–5045. doi: 10.1038/sj.onc.1209513. [DOI] [PubMed] [Google Scholar]
- Chatterjee SJ, George B, Goebell PJ, Alavi-Tafreshi M, Shi SR, Fung YK, et al. Hyperphosphorylation of pRb: a mechanism for RB tumour suppressor pathway inactivation in bladder cancer. J Pathol. 2004;203:762–770. doi: 10.1002/path.1567. [DOI] [PubMed] [Google Scholar]
- Christian BJ, Loretz LJ, Oberley TD, Reznikoff CA. Characterization of human uroepithelial cells immortalized in vitro by simian virus 40. Cancer Res. 1987;47:6066–6073. [PubMed] [Google Scholar]
- Cooper CS, Nicholson AG, Foster C, Dodson A, Edwards S, Fletcher A, et al. Nuclear overexpression of the E2F3 transcription factor in human lung cancer. Lung Cancer. 2006;54:155–162. doi: 10.1016/j.lungcan.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, Wartinger D, Petrylak D, Dalbagni G, Fair WR, Fuks Z, et al. Altered expression of the retinoblastoma gene product: prognostic indicator in bladder cancer. Journal of The National Cancer Institute. 1992;84:1251–1256. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- Cote RJ, Dunn MD, Chatterjee SJ, Stein JP, Shi SR, Tran QC, et al. Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res. 1998;58:1090–1094. [PubMed] [Google Scholar]
- DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci U S A. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. doi: 10.1038/sj.onc.1208612. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Evans AJ, Gallie BL, Jewett MA, Pond GR, Vandezande K, Underwood J, et al. Defining a 0.5-mb region of genomic gain on chromosome 6p22 in bladder cancer by quantitative-multiplex polymerase chain reaction. Am J Pathol. 2004;164:285–293. doi: 10.1016/S0002-9440(10)63118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feber A, Clark J, Goodwin G, Dodson AR, Smith PH, Fletcher A, et al. Amplification and overexpression of E2F3 in human bladder cancer. Oncogene. 2004;23:1627–1630. doi: 10.1038/sj.onc.1207274. [DOI] [PubMed] [Google Scholar]
- Fiegler H, Carr P, Douglas EJ, Burford DC, Hunt S, Smith J, et al. DNA microarrays for comparative genomic hybridization based on DOP-PCR amplification of BAC and PAC clones. Genes Chromosomes Cancer. 2003;36:361–374. doi: 10.1002/gcc.10155. [DOI] [PubMed] [Google Scholar]
- Frolov MV, Huen DS, Stevaux O, Dimova D, Balczarek-Strang K, Elsdon M, et al. Functional antagonism between E2F family members. Genes Dev. 2001;15:2146–2160. doi: 10.1101/gad.903901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Armanious MK, Thomas MJ, Cress WD. Identification of E2F-3B, an alternative form of E2F-3 lacking a conserved N-terminal region. Oncogene. 2000;19:3422–3433. doi: 10.1038/sj.onc.1203682. [DOI] [PubMed] [Google Scholar]
- Humbert PO, Verona R, Trimarchi JM, Rogers C, Dandapani S, Lees JA. E2f3 is critical for normal cellular proliferation. Genes Dev. 2000;14:690–703. [PMC free article] [PubMed] [Google Scholar]
- Hurst CD, Fiegler H, Carr P, Williams S, Carter NP, Knowles MA. High-resolution analysis of genomic copy number alterations in bladder cancer by microarray-based comparative genomic hybridization. Oncogene. 2004;23:2250–2263. doi: 10.1038/sj.onc.1207260. [DOI] [PubMed] [Google Scholar]
- Kallioniemi A, Kallioniemi O-P, Citro G, Sauter G, DeVries S, Kerschmann R, et al. Identification of gains and losses of DNA sequences in primary bladder cancer by comparative genomic hybridisation. Genes Chromosomes and Cancer. 1995;12:213–219. doi: 10.1002/gcc.2870120309. [DOI] [PubMed] [Google Scholar]
- Lazzerini Denchi E, Attwooll C, Pasini D, Helin K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol Cell Biol. 2005;25:2660. doi: 10.1128/MCB.25.7.2660-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Frere-Belda MA, Gil Diez de Medina S, Daher A, Martin N, Albaud B, Heudes D, et al. Profiles of the 2 INK4a gene products, p16 and p14ARF, in human reference urothelium and bladder carcinomas, according to pRb and p53 protein status. Hum Pathol. 2004;35:817. doi: 10.1016/j.humpath.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Lees JA, Saito M, Vidal M, Valentine M, Look T, Harlow E, et al. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993;13:7813–7825. doi: 10.1128/mcb.13.12.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone G, DeGregori J, Yan Z, Jakoi L, Ishida S, Williams RS, et al. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 1998;12:2120–2130. doi: 10.1101/gad.12.14.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone G, Nuckolls F, Ishida S, Adams M, Sears R, Jakoi L, et al. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol Cell Biol. 2000;20:3626–3632. doi: 10.1128/mcb.20.10.3626-3632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis CJ, Xu H-J, Ro JY, Hu S-X, Sahin A, Ordonez N, et al. Altered expression of retinoblastoma protein and known prognostic variables in locally advanced bladder cancer. Journal of The National Cancer Institute. 1992;84:1256–1261. doi: 10.1093/jnci/84.16.1256. [DOI] [PubMed] [Google Scholar]
- Oeggerli M, Schraml P, Ruiz C, Bloch M, Novotny H, Mirlacher M, et al. E2F3 is the main target gene of the 6p22 amplicon with high specificity for human bladder cancer. Oncogene. 2006;25:6538–6543. doi: 10.1038/sj.onc.1209946. [DOI] [PubMed] [Google Scholar]
- Oeggerli M, Tomovska S, Schraml P, Calvano-Forte D, Schafroth S, Simon R, et al. E2F3 amplification and overexpression is associated with invasive tumor growth and rapid tumor cell proliferation in urinary bladder cancer. Oncogene. 2004;23:5616–5623. doi: 10.1038/sj.onc.1207749. [DOI] [PubMed] [Google Scholar]
- Olsson AY, Feber A, Edwards S, Te Poele R, Giddings I, Merson S, et al. Role of E2F3 expression in modulating cellular proliferation rate in human bladder and prostate cancer cells. Oncogene. 2007;26:1028–1037. doi: 10.1038/sj.onc.1209854. [DOI] [PubMed] [Google Scholar]
- Orlic M, Spencer CE, Wang L, Gallie BL. Expression analysis of 6p22 genomic gain in retinoblastoma. Genes Chromosomes Cancer. 2006;45:72–82. doi: 10.1002/gcc.20263. [DOI] [PubMed] [Google Scholar]
- Paulie S, Hansson Y, Lundblad ML, Perimann P. Lectins as probes for identification of tumor-associated antigens on urothelial and colonic carcinoma cell lines. International Journal of Cancer. 1983;31:297–303. doi: 10.1002/ijc.2910310308. [DOI] [PubMed] [Google Scholar]
- Paulson QX, McArthur MJ, Johnson DG. E2F3a stimulates proliferation, p53-independent apoptosis and carcinogenesis in a transgenic mouse model. Cell Cycle. 2006;5:184–190. doi: 10.4161/cc.5.2.2307. [DOI] [PubMed] [Google Scholar]
- Prat E, Bernues M, Caballin MR, Egozcue J, Gelabert A, Miro R. Detection of chromosomal imbalances in papillary bladder tumors by comparative genomic hybridization. Urology. 2001;57:986–992. doi: 10.1016/s0090-4295(01)00909-8. [DOI] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- Stevens C, La Thangue NB. The emerging role of E2F-1 in the DNA damage response and checkpoint control. DNA Repair (Amst) 2004;3:1071–1079. doi: 10.1016/j.dnarep.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. Embo J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson DC, Hurst CD, Knowles MA. Knockdown by shRNA identifies S249C mutant FGFR3 as a potential therapeutic target in bladder cancer. Oncogene. 2007;26:5889–5899. doi: 10.1038/sj.onc.1210399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomovska S, Richter J, Suess K, Wagner U, Rozenblum E, Gasser TC, et al. Molecular cytogenetic alterations associated with rapid tumor cell proliferation in advanced urinary bladder cancer. Int J Oncol. 2001;18:1239–1244. doi: 10.3892/ijo.18.6.1239. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Veltman JA, Fridlyand J, Pejavar S, Olshen AB, Korkola JE, DeVries S, et al. Array-based comparative genomic hybridization for genome-wide screening of DNA copy number in bladder tumors. Cancer Res. 2003;63:2872–2880. [PubMed] [Google Scholar]
- Wu Q, Hoffmann MJ, Hartmann FH, Schulz WA. Amplification and overexpression of the ID4 gene at 6p22.3 in bladder cancer. Mol Cancer. 2005;4:16. doi: 10.1186/1476-4598-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HJ, Cairns P, Hu SX, Knowles MA, Benedict WF. Loss of RB protein expression in primary bladder cancer correlates with loss of heterozygosity at the RB locus and tumor progression. Int J Cancer. 1993;53:781–784. doi: 10.1002/ijc.2910530513. [DOI] [PubMed] [Google Scholar]
- Yeager T, Stadler W, Belair C, Puthenveettil J, Olopade O, Reznikoff C. Increased p16 levels correlate with pRB alterations in human urothelial cells. Cancer Research. 1995;55:493–497. [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebold U, Lee EY, Bronson RT, Lees JA. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol Cell Biol. 2003;23:6542–6552. doi: 10.1128/MCB.23.18.6542-6552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebold U, Reza T, Caron A, Lees JA. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 2001;15:386–391. doi: 10.1101/gad.858801. [DOI] [PMC free article] [PubMed] [Google Scholar]