Abstract
Maintaining cell shape and tone is crucial for the function and survival of cells and tissues. Mechanotransduction relies on the transformation of minuscule mechanical forces into high-fidelity electrical responses1 2 3. When mechanoreceptors are stimulated, mechanically sensitive cation channels open and produce an inward transduction current that depolarizes the cell. For this process to operate effectively, the transduction machinery has to retain integrity and remain unfailingly independent of environmental changes. This is particularly challenging for poikilothermic organisms, where changes in temperature in the environment may impact the function of mechanoreceptor neurons. Thus, we wondered how insects whose habitat might quickly vary over several tens of degrees of temperature manage to maintain highly effective mechanical senses. We screened for Drosophila mutants with defective mechanical responses at elevated ambient temperatures, and identified a gene, spam, whose role is to protect the mechanosensory organ from massive cellular deformation caused by heat-induced osmotic imbalance. Here, we show that Spam protein forms an extracellular shield that guards mechanosensory neurons from environmental insult. Remarkably, heterologously expressed Spam protein also endowed other cells with superb defense against physically- and chemically-induced deformation. We studied the mechanical impact of Spam-coating and show that spam-coated cells are up to ten times stiffer than uncoated-controls. Together, these results help explain how poikilothermic organisms preserve the architecture of critical cells during environmental stress, and illustrate an elegant and simple solution to such challenge.
Fly mechanoreceptor neurons (MRNs) are essential for a number of critical functions such as hearing, proprioception, flight control and touch sensing, and their mis-function leads to uncoordination and loss of mechanoreceptor responses4 5. To identify components of the machinery that preserve the functional integrity of the mechanosensory apparatus at high environmental temperatures, we carried out a genetic screen for temperature-sensitive uncoordinated flies; we anticipated that loss-of-function mutations in such components may render MRN function highly susceptible to the elevated temperature. Approximately 12,000 ethylmethane sulphonate-mutagenized homozygous lines6 were examined for intact locomotor responses at RT, but defective behavior after 1 hr at 37°C . One mutant line, 2649, had no apparent defects at RT, including walking, feeding and flying, but upon shifting to the restrictive temperature the flies gradually lost the ability to fly, to stand upside-down, and to climb the walls, until eventually they could only lie and sporadically move their legs, wings and mouth-parts in an uncoordinated manner (Supplementary Fig. 1 and Supplementary videos). Genetic mapping and transformation rescue experiments proved that the mechanosensory defects of line 2649 are due to a non-sense mutation in the spacemaker gene (spam; see Fig. 1).
Figure 1. Effect of heat exposure on the function of mechanoreceptor cells.
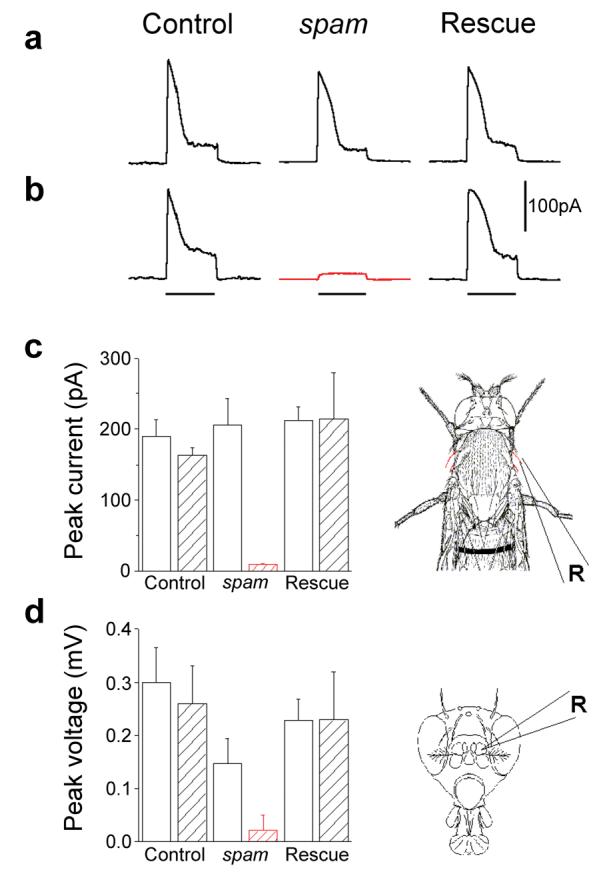
a-b, MRN responses from a single voltage-clamped bristle to mechanical stimuli. Upper panel (left to right): responses of control (cn bw flies), spam (spam/spam mutants) and rescue flies (spam homozygotes expressing a wild-type spam transgene). Note normal responses of all 3 samples at 21°C. b, After incubation for 30 minutes at 37°C (lower panel), responses were abolished in spam mutants (red trace). Lines under the traces indicate the 0.3 sec. duration of a 30μm deflection stimuli. c, Summary of peak responses of a-b (diagram to the right illustrate site of recordings). d, Summary of peak extracellular voltage responses to antennal rotation (pipette position illustrated on the right diagram). Open columns, 21°C; hatched columns, responses after 30 min at 37°C. R illustrates the position of the recording pipette. Error bars indicate standard deviation (n≥6 for each trial).
Recently, we showed that Spam encodes an extracellular protein required for creating the intra-rhabdomeral space (IRS) in the compound eyes of insects with open rhabdom systems7. There, Spam provides the extracellular substrate to sustain the precise arrangement of rhabdomeres within each ommatidium7. Notably, the other sites of Spam expression are on mechanosensory and chemosensory neurons8. To directly examine the impact of loss-of-function mutations in spam on mechanosensory transduction, we performed electrophysiological recordings from bristle mechanoreceptors (touch)9 and antennal chordotonal organs (hearing)10 from control and mutant flies. We gave sensory bristles calibrated mechanical stimuli while recording transduction currents with a voltage-clamp apparatus. At 21°C, control flies and spam mutants displayed robust inward currents in response to bristle deflections (Fig. 1a-c). In contrast, 30 min of exposure to 37°C reduced mechanoreceptor response amplitudes in spam mutant animals by over 80%. The same heat exposure also nearly abolished all mechanoreceptor antennal responses, while having no significant effect on control flies (Fig. 1d).
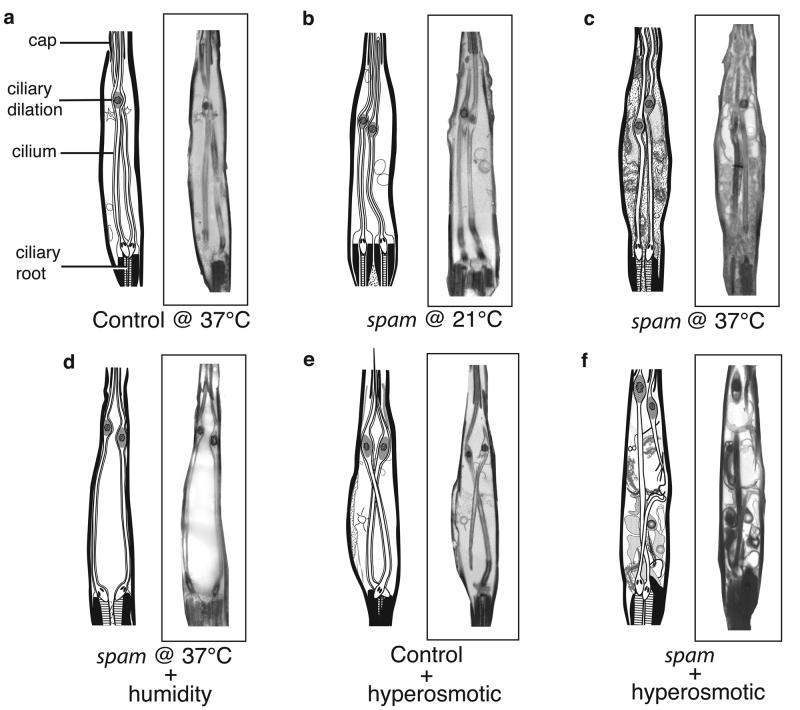
Next, we examined the ultrastructure of MRN in control flies and spam mutants at both permissive and non-permissive temperatures. Drosophila mechano- and chemosensory neurons house their entire sensory apparatus in a ciliated outer-segment that forms the neuronal sensory endings11. In the case of MRNs, this outer segment is bathed in an extracellular fluid (lymph) which provides the proper ionic environment for the generation of mechanoreceptor currents11. Remarkably, spam mutants, but not control flies, experience a dramatic deformation of their MRN in response to heat treatment: the entire neuronal cytoplasm invades the lymph space, such that the region that normally contained only the cilium and extracellular fluid now becomes filled with cellular material from the MRN cell body (compare figures 2a,b versus c; see also Supplementary Fig. 2).
Figure 2. Mechanoreceptors of spam mutants undergo dramatic cellular deformation.
Electron micrographs of a typical scolopale MRN in the Johnston's organ. (a) control (cn bw) flies at 37°C and (b) spam homozygous mutants at 21°C have nearly indistinguishable morphology (equivalent results are observed with cn bw flies at 21°C). However, (c) exposure of spam flies to 30 min at 37°C results in major cellular deformation, with the receptor cell cytoplasm expanding to fill the entire scolopale space (importantly, the cells that wrap around the scolopale space are unaffected; data not shown). (d) Placing spam mutants in a high-humidity chamber (>90% RH) prevents the heat-induced deformation. (e-f) cn bw control and spam flies injected with a hyper-osmotic solution to the abdomen. Only spam mutants display dramatic cellular deformation with extensive invasion of the extracellular space. Note that some of the reconstructed EM micrographs show a side view of the scolopale, with only one ciliary root visible (panels a and e), while all others show a front view, with both ciliary roots visible.
How does exposure to elevated temperatures have such a dramatic effect on the morphology of spam MRNs? Changes in molecular thermal motion between 21°C and 37°C are too small, and unlikely to account for the phenotype. We therefore considered a prominent secondary effect of heat: water loss by evaporation. To investigate how much water is lost during the heat exposure, we measured the weight of control and mutant flies at 15 min intervals. All flies lose ∼20% of their total weight after 60 min at 37°C (∼25% of their water content; data not shown), yet only the mutants display the mechanosensory defect. To determine whether the heat-induced deformation of MRN in spam mutants is indeed a consequence of water loss, we placed spam flies either in a control petri dish or in a dish at over 90% humidity, and subjected them to the 60 minutes treatment at 37°C. Notably, only the flies in the dry chamber were affected by heat; exposure to high humidity during the high-temperature treatment completely prevented the manifestation of the mutant phenotype, both morphologically (Fig. 2c,d) and behaviorally (Supplementary material, compare videos 4 and 6). These data demonstrate that the mutant's mechanosensory deficit does not arise from an effect of temperature per se, but is instead triggered by excessive water evaporation at high temperature12. Why does water loss lead to deformation of the MRN only in spam mutants? We hypothesized that the rapid loss of water from the animal's circulatory system (hemolymph) would increase its osmolarity, leading to an outflow of water from the sensory lymph. The new imbalance between the MRN cytoplasm and the lymph would cause the deformation of the MRN cytosol, which if not contained (as in the absence of Spam protein; see below), would then invade the lymph space. This proposed mechanism anticipates that hypertonic shock to the hemolymph of spam mutants, but not wild type animals, should mimic the effect of high temperature on the morphology and function of MRNs (i.e. hypertonic shock should induce a similar osmotic imbalance between the endolymph and the MRN cytoplasm). We injected a high-osmolarity solution to the abdomen of spam and control flies and prepared them for EM examination. As hypothesized, only spam flies showed deformation of the MRN (Fig. 2e,f) and loss of mechanosensory responses (Supplementary Fig. 3), substantiating the mechanism of deformation and the role of Spam in maintaining cell-shape.
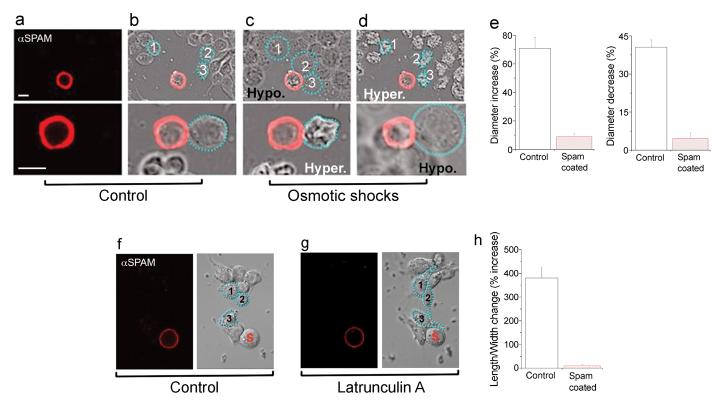
In photoreceptor neurons, Spam is secreted into the inter-rhabdomeral space where it forms the extracellular medium that organizes and preserves the separation of rhabdomeres. We reasoned that in mechanoreceptor neurons the role of Spam might be a variation on this theme, perhaps this time serving as a cellular exoskeleton that provides structural rigidity to the MRN, thus ensuring the preservation of cell shape under environmental stress. This postulate makes two significant predictions: First, Spam protein should be specifically localized within the fly's mechanoreceptor organ, at locations that might be particularly vulnerable to osmotic pressure changes. Second, if Spam functions as a mechanical barrier that protects MRN from deformation, it should be possible to engineer cells that are coated with Spam and make them resistant to osmotic insult and deformation pressures. Indeed, Spam protein concentrates at two specific sites in MRN: one, right at the interface between the MRN cell body and the lymph space, the very domain that collapses at high temperature in mutant animals (see Supplementary Fig. 4 d, e), and at a second site close to the ciliary dilation, possibly helping sustain the two ciliary processes at the proper position (Supplementary Fig. 4). To generate cells that are decorated by a layer of Spam, we took advantage of Spam's ability to directly bind the membrane receptor Prominin7. Therefore, Drosophila tissue culture cells expressing and secreting Spam were incubated with GFP-labeled cells transfected with Prominin. As expected, secreted Spam specifically decorated the surface of Prominin-expressing cells; to identify those cells that are entirely (or nearly completely) coated, we performed immunofluorescent staining with anti-Spam antibodies. We induced cellular deformation by subjecting control and coated-cells to hyper- and hypo-osmotic solutions. As predicted, control cells undergo significant swelling following hypo-osmotic shock, and severe shrinking in the presence of hyper-osmotic solutions (Fig. 3a-e). In contrast, coated cells were largely resistant to these treatments and showed only minor changes in shape and size (not surprisingly, poorly coated cells were indistinguishable from controls; data not shown). Next, we examined the impact of Spam on chemically-induced cell shape changes13. We subjected control cells to latrunculin A and elicited dramatic changes in cell morphology (Fig. 3f-h). However, Spam coated cells retained their normal spherical shape, even after extensive actin remodeling resulting from the latrunculin A treatment (see Methods). Collectively, these studies demonstrate that Spam coating of the plasma membrane endows cells with exquisite protection against osmotically- and chemically-induced transformations in cell shape.
Figure 3. Spam coating prevents cell deformation induced by osmotic or chemical manipulation.
(a-d) Kc tissue culture cells transfected with Prom and Spam were stained for Spam surface labeling (red) using anti-Spam antibodies on unpermeabilized, intact cells. Spam-coated (panel a) and uncoated cells were then subjected to osmotic shock. (b-d) Upper panels show low magnification images of cells before and after sequential hypo- and hyper-osmotic shock; lower panels show similar cells at higher magnification but this time after sequential hyper- and then hypo-osmotic shock (reverse order). Hypo-osmotic shock causes dramatic swelling of uncoated cells, while hyper-osmotic treatment of the same preparation leads to extreme shrinking. Notably, the spam-coated cell remains largely unaffected by both treatments. All cells that showed a continuous layer of Spam coating (> 0.5μm thick layer and with no apparent gaps) showed no significant shape changes in response to the osmotic shocks (n=11), while all uncoated cells displayed severe changes in size and shape (n>150). (e) Bar graphs illustrating the increases (left panel) and decreases (right panel) in cell size following hypotonic or hypertonic shock; control, n≥24, spam-coated, n≥8; error bars denote SEM. (f) Spam-coated (red) and uncoated cells were incubated for 90 minutes with latrunculin A. (g) As expected, control cells undergo dramatic changes in cell shape13. In contrast, the spam-coated cell (S) remains unaltered. Blue dots and numbers delineate the shape of three sample uncoated cells before (panel f) and after treatment (panel g). Images were captured using epifluorescence and Nomarski interference contrast. (h) Bar graphs illustrating the changes in cell shape (defined as changes in the ratio of cell length over width) following latrunculin treatment in Spam-coated (n=9) and control uncoated cells (n=27); error bars denote SEM.
How robust are Spam-treated cells? We directly examined the stiffness of Spam-coated and control cells by measuring their mechanical properties. In these experiments, a glass filament of known bending constant is continuously pressed against the cell using a linear piezoelectric drive14 (Fig. 4a-b). The force applied to the tip of the probe by the resistance of the cell to indentation is then calculated by optically measuring the bending of the glass probe. The major source of stiffness in cells is the actin cytoskeleton15. Therefore, to eliminate the contribution of the cystoskeleton and explore the specific effect of Spam, experimental and control samples were first treated with cytochalasin D for 120 min. The results (Fig. 4c) demonstrate that Spam-coated cells exhibit stiffness that is approximately 10 times that of control cells.
Figure 4. Mechanical impact of spam coating.
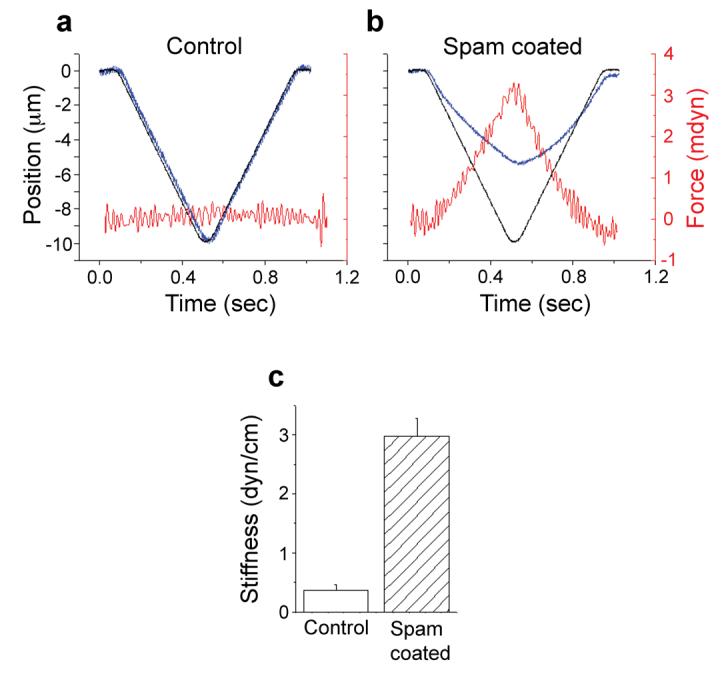
To measure the stiffness of Spam-coated cells, (a) control or (b) Spam-coated tissue culture cells were subjected to a mechanical indentation assay14. In order to reduce the contribution of the cystoskeleton to cell stiffness (and thereby reveal the effect of Spam coating more effectively), samples were pre-treated with cytochalasin D as previously described15. (a-b) The diagrams compare the position over time of the motor that moves the probe assembly (black trace) versus the position of the stylus that indents the cell (blue trace). The difference between the two curves at a given time is due to the cell's resistance to indentation. The force applied by the cell against the probe is proportional to this difference and is shown in red. (c) Stiffness (cell resistance force per unit indentation) was calculated as described14. A minimum of 9 individual cells were examined for each experiment; error bars indicate SEM. Control cells without cytochalasin D are presented in Supplementary Figure 5.
Together, these studies have revealed a remarkable solution to the problem of maintaining cellular integrity and structure under duress. They also provide a salient example of evolution employing the same protein to satisfy two very different needs: the building of compound eyes in open rhabdom systems16 7, and the preservation of cell shape in mechano- and chemoreceptor organs. Interestingly, both entail the production and assemblage of a rigid substrate, thus highlighting the fundamental role of Spam in tissue morphogenesis (in one scenario to ensure the partitioning and maintenance of the rhabdomere complex, and in the other to guarantee the mechanical integrity of sensory neurons). Finally, it is worth noting that the ability to assemble a “cell-wall” surrounding an animal cell may provide the foundation for important applications in cell engineering, where resistance to osmotic pressures may be warranted, or where preservation of cell and tissue structure (or tone) may be needed.
METHODS SUMMARY
Fly stocks
An isogenized cn bw stock was used as control in all experiments. The spam line was isolated from the Zuker collection6 and the rescue was done using hs-gal4 driving UAS-spam7.
Electrophysiology
Single bristle current recordings were performed as described earlier9. Voltage changes resulting from Johnston's organ activation were monitored by inserting a glass pipette (2M KCl, ∼10MΩ) into the second antennal segment. Mechanical stimulation was delivered by a stream of air that was directed at the arista causing a rotation of the third segment for the duration of air flow.
In vivo osmotic manipulation
Flies were glued ventral side up and manually injected using a glass pipette with a tip of 20-40μm. After 10 min, tissue was prepared for analysis as described under Electron microscopy.
Electron microscopy
Heads of 7-10 days old flies were fixed and sectioned exactly as previously described7. A series of coronal sections (100-200nm per section) through the antennal second segment were obtained. The entire scolopale was reconstructed from overlapping sections using Adobe imaging software.
Tissue culture
Kc cells13 were transfected with combinations of pTub-GAL4 and pUAST-spacemaker, pUAST-prominin, and pUAST-GFP, as previously described7. Spam coating was detected in vivo using its specific antibody mAb21A617. Hypo-osmotic shock was induced by diluting the growth medium 1:5x with distilled water; hyper-osmotic conditions were obtained by adding 50μl of 5M NaCl to 1.25 ml of growth media. Latrunculin A (Sigma) was used at a final concentration of 0.2 μM18.
Cell indentation assay
KC cells co-transfected with pTub-GAL4, pUAST-spacemaker, and pUAST-prominin were treated with 2μM cytochalasin D for at least 100 min and the Spam-coated cells identified by labeling with anti-spam antibodies. Indentation tests were performed on control and Spam-coated cells as previously described14.
METHODS
Fly stocks
An isogenized cn bw stock was used as control in all experiments. The spam line was isolated from the Zuker collection6 and the rescue was done using hs-gal4 driving UAS-spam7.
Electrophysiology
Single bristle current recordings were performed as described earlier9. Voltage changes resulting from Johnston's organ activation were monitored by inserting a glass pipette (2M KCl, ∼10MΩ) into the second antennal segment. Mechanical stimulation was delivered by a stream of air that was directed at the arista causing a rotation of the third segment for the duration of air flow. Signals were acquired using an EX1 differential amplifier (DAGAN) and a pCLAMP (Axon Instruments) system, and analyzed using Origin (Microcal Software).
In vivo osmotic manipulation
Flies were glued ventral side up and manually injected using a glass pipette with a tip of 20-40μm. A volume of 0.2μl of a solution containing 1M mannitol was injected into the abdomens of male and female control or spam mutant flies. After 10 min, tissue was prepared for analysis as described under Electron microscopy.
Electron microscopy
Heads of 7-10 days old flies were fixed and sectioned exactly as previously described7. A series of coronal sections (100-200nm per section) through the antennal second segment were obtained and examined using a JEOL 1200EX II or a Philips CM-10 transmission electron microscopes; at least 3 flies from a minimum of 2 independent experiments were examined. The entire scolopale was reconstructed from overlapping sections using Adobe imaging software.
Tissue culture
In order to coat cells with a layer of Spam, we took advantage of its selective binding to Prominin, the Spam receptor in photoreceptor cells7. The nature of the Spam receptor in MRNs, if any, is not yet known. Kc cells13 were transfected with combinations of pTub-GAL4 and pUAST-spacemaker, pUAST-prominin, and pUAST-GFP, as previously described7. Spam coating was detected in vivo using 45 min incubation with mAb21A617 (1:100 in growth medium), followed by a 45min incubation with Red-x-conjugated secondary antibody (Jackson ImmuoResearch Laboratories). Hypo-osmotic shock was induced by diluting the growth medium 1:5x with distilled water; hyper-osmotic conditions were obtained by adding 50μl of 5M NaCl to 1.25 ml of growth media. Vital staining was performed by addition of Trypan Blue (0.04% final concentration, GIBCO) to the growth medium. Latrunculin A (Sigma) was used at a final concentration of 0.2 μM18 to prevent polymerization of actin. To ensure that latrunculin A was effective in disrupting actin cytoarchitecture, control and Spam-coated cells were labeled with fluorescein isothiocyanate (FITC) conjugated phalloidin (Invitrogen). In all cases, Latrunculin A induced the formation of F-actin aggregates (data not shown). Time lapse imaging of transmitted and fluorescent signals were performed using either a BioRad MRC1024 or an Olympus FluoView1000 confocal microscope. Changes in cell size in the hypotonic shock studies were analyzed by measuring the cell's diameter before and after osmotic shock. Changes in cell shape in the latrunculin experiments were quantified by using the ratio between the cell's length (defined as its longest axis) and its width (perpendicular to the length) before and after drug treatment. Cell measurements were performed using standard imaging software and data was analyzed using Origin Software.
Cell indentation assay
KC cells co-transfected with pTub-GAL4, pUAST-spacemaker, and pUAST-prominin were treated with 2μM cytochalasin D for at least 100 min (to interrupt F-actin formation by capping the barbed ends), and the Spam-coated cells identified by labeling with anti-spam antibodies. Indentation tests were performed on control and Spam-coated cells as previously described14 using a probe with a spring constant of 6.72 dyne/cm and tip diameter of 5 μm. Data analysis was performed exactly as described previously, with stiffness (K) defined as K = G(1-(dM/dt)/(dP/dt), M(t)= motor position, P(t)= tip position, G=probe stiffness; this calculation does not take into account inertia and viscous drag14. Probe control, data collection and analysis were performed using the Experix environment (https://sourceforge.net/projects/experix).
Supplementary Material
Acknowledgements
We particularly thank Elliot L. Elson for his help and hospitality in the execution of the cell poking studies. We are also indebted to Andrew Zelhof for advice, materials and reagents, and Tomer Avidor-Reiss for his generous help with the immuno-localization of Spam. We thank Timo Meerloo for help with immunogold labeling, and Ann Becker for help with tissue culture and transfections. We thank Nick Ryba, Amy Kiger and members of the Zuker lab for helpful comments. CSZ is an investigator of the Howard Hughes Medical Institute.
References
- 1.Colclasure JC, Holt JR. Transduction and adaptation in sensory hair cells of the mammalian vestibular system. Gravit Space Biol Bull. 2003;16:61–70. [PubMed] [Google Scholar]
- 2.Hudspeth AJ. How the ear's works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 3.Barth ,FG. Spider mechanoreceptors. Curr Opin Neurobiol. 2004;14:415–22. doi: 10.1016/j.conb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 5.Kernan M, Zuker C. Genetic approaches to mechanosensory transduction. Curr Opin Neurobiol. 1995;5:443–8. doi: 10.1016/0959-4388(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 6.Koundakjian EJ, Cowan DM, Hardy RW, Becker AH. The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics. 2004;167:203–6. doi: 10.1534/genetics.167.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelhof AC, Hardy RW, Becker A, Zuker CS. Transforming the architecture of compound eyes. Nature. 2006;443:696–9. doi: 10.1038/nature05128. [DOI] [PubMed] [Google Scholar]
- 8.Husain N, et al. The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev Cell. 2006;11:483–93. doi: 10.1016/j.devcel.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–34. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 10.Gopfert MC, Robert D. Biomechanics. Turning the key on Drosophila audition. Nature. 2001;411:908. doi: 10.1038/35082144. [DOI] [PubMed] [Google Scholar]
- 11.Keil TA. Functional morphology of insect mechanoreceptors. Microsc Res Tech. 1997;39:506–31. doi: 10.1002/(SICI)1097-0029(19971215)39:6<506::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs AG, Louie AK, Ayala JA. Effects of temperature on cuticular lipids and water balance in a desert Drosophila: is thermal acclimation beneficial? J Exp Biol. 1998;201:71–80. doi: 10.1242/jeb.201.1.71. [DOI] [PubMed] [Google Scholar]
- 13.Kiger AA, et al. A functional genomic analysis of cell morphology using RNA interference. J Biol. 2003;2:27. doi: 10.1186/1475-4924-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahalak GI, McConnaughey WB, Elson EL. Determination of cellular mechanical properties by cell poking, with an application to leukocytes. J Biomech Eng. 1990;112:283–94. doi: 10.1115/1.2891186. [DOI] [PubMed] [Google Scholar]
- 15.Wakatsuki T, Schwab B, Thompson NC, Elson EL. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J Cell Sci. 2001;114:1025–36. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- 16.Osorio D. Spam and the evolution of the fly's eye. Bioessays. 2007;29:111–5. doi: 10.1002/bies.20533. [DOI] [PubMed] [Google Scholar]
- 17.Zipursky SL, Venkatesh TR, Teplow DB, Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984;36:15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]
- 18.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–44. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.