Abstract
BACKGROUND
Successful blastocyst implantation requires the differentiation of human endometrial stromal cells (HESC), a process known as decidualization. Activin A, a transforming growth factor β (TGFβ) superfamily member, enhances HESC decidualization and localizes to decidual cells in human endometrium. Other TGFβ superfamily members, including BMP2, BMP4, BMP7, GDF5, GDF8, GDF11, TGFβs and Nodal, may also play a role during decidualization. This study aimed to identify these TGFβ family members in human endometrium, and to determine whether they are involved in human decidualization.
METHODS
Protein localization of TGFβ family members was examined in secretory phase human endometrium and first trimester decidua by immunohistochemistry. mRNA expression was examined in HESC. Activin inhibitors (Activin-M108A/SB431542) with differing specificities for the other TGFβ members under consideration were applied during HESC decidualization in vitro. The secretion levels of potential TGFβ superfamily members were measured during decidualization, and recombinant proteins added to examine their effect.
RESULTS
This study has identified BMP2, BMP4, BMP7, GDF5, GDF8 and GDF11 but not Nodal in secretory phase human endometrium, but only BMP2, GDF5 and TGFβ1 protein were detected in decidual cells. All ligands except Nodal were expressed by cultured HESC. Both inhibitors significantly reduced decidualization validating the role of activin, but potentially also other TGFβ members, during decidualization. BMP2 and TGFβ1 secretion increased during HESC decidualisation and exogenous administration of these proteins significantly enhanced decidualization in vitro.
CONCLUSIONS
Like activin, BMP2 and TGFβ1 are likely to be involved in HESC decidualization. This is the first study to identify and localize BMP4, BMP7, GDF5, GDF8 and GDF11 in secretory phase human endometrium. Understanding the factors critical for the implantation process is needed for improving fertility and pregnancy outcomes.
Keywords: human endometrium; growth factors (activins, BMP, TGFβ); implantation; decidualization
Introduction
Throughout each menstrual cycle the human endometrium undergoes morphological and physiological changes, in preparation for pregnancy should the cycle include conception (Loke et al., 1995). An absolute requirement for successful implantation is the differentiation and proliferation of endometrial stromal cells into enlarged, phenotypically different decidual cells, a process termed endometrial decidualization (Tang et al., 1994; Salamonsen et al., 2003). This process is initiated near the spiral arterioles (Bell, 1991), and occurs under the influence of progesterone (Psychoyos, 1973), although cAMP appears to be essential for priming the endometrial stromal cells to the actions of progesterone; hence both pathways are absolutely required for decidualization (Gellersen and Brosens, 2003). It is now clear that the decidualization response is mediated by a complex array of bioactive molecules, including IL-11, prostaglandin E2, relaxin and activins (Frank et al., 1994; Robb et al., 1998; Jones et al., 2002; Dimitriadis et al., 2005).
The transforming growth factor β (TGFβ) superfamily is a large family of proteins that encompasses the sub-families TGFβs, activins, bone morphogenetic proteins (BMPs) and growth differentiation factors (GDFs). Overall, this superfamily of proteins exhibits functional diversity, with biological roles in cell differentiation, proliferation, apoptosis and tissue remodelling, all consistent with various reproductive processes (Jones et al., 2006).
Activins consist of two β subunits, βA and βB, that homo/heterodimerize to form activin A, activin B and activin AB, respectively. The activin β subunits are produced in the glandular epithelium during the proliferative and secretory phases, but in stromal cells only following decidualization during the mid-late secretory phase (Otani et al., 1998; Jones et al., 2000; Mylonas et al., 2004). High levels of dimeric activin A are secreted by cAMP-treated endometrial stromal cells. Addition of activin A to decidualizing cells in vitro significantly increased the secretion of prolactin (PRL) and IGFBP-1 (decidual cell markers), suggesting activin A drives decidualization (Jones et al., 2002; Tierney and Giudice, 2004). This decidualization response can be neutralized by follistatin, the naturally occurring activin antagonist (Jones et al., 2002; Tierney and Giudice, 2004). In addition to its tight regulation of activin (Shimonaka et al., 1991; Schneyer et al., 1994), follistatin can also bind and regulate other TGFβ members including the BMPs and GDFs (Table I).
Table I.
Inhibitors of TGFβ superfamily: ligand–receptor blocking.
| Ligand | Follistatin | Activin-M108A | SB431542 |
|---|---|---|---|
| Binds ligands and blocks association with type II receptors | Activin type II receptor (ActRIIA/B) antagonist | Inhibitor of TGFβ and activin type I receptors | |
| Activin A | ++++ (Schneyer et al., 2003) | ++ (Harrison et al., 2004) | +++ (Inman et al., 2002) |
| Activin B | ++ (Schneyer et al., 2003) | +++a | +++ (Inman et al., 2002) |
| GDF 8 | ++ (Amthor et al., 2004) | +++a | +++ (Inman et al., 2002) |
| GDF11 | ++ (Gamer et al., 1999) | +++a | +++ (Inman et al., 2002) |
| BMP2 | + (Iemura et al., 1998) | − | − |
| BMP4 | + (Iemura et al., 1998; Glister et al., 2004) | − | − |
| BMP7 | + (Iemura et al., 1998; Glister et al., 2004) | (−/+) | − |
| GDF5 | (+) | − | − |
| Nodal | − | (+++) | +++ (Inman et al., 2002) |
| TGFβ1 | − | − | +++ (Inman et al., 2002) |
| TGFβ2 | − | − | +++ (Inman et al., 2002) |
| TGFβ3 | − | − | +++ (Inman et al., 2002) |
aC.A. Harrison (unpublished observations); (), anticipated activity.
BMPs have been identified in the rodent uterus (Cunningham et al., 1995; Ozkaynak et al., 1997; Fitzpatrick et al., 1998; Zhao et al., 1999; Ying and Zhao, 2000; Erickson et al., 2004; Lee et al., 2007; Li et al., 2007). In pregnant mice the temporospatial localization of BMPs 2, 4, 6 and 7 suggested roles during pregnancy (Ying and Zhao, 2000). BMP2 is present within the decidual area at implantation sites (Ying and Zhao, 2000; Li et al., 2007) and plays a role in decidualization in vitro (Li et al., 2007). Importantly, conditional ablation of uterine BMP2 in mice resulted in ineffective decidualization and disrupted pregnancy outcome (Lee et al., 2007). In a human model of endometrial stromal cell (HESC) decidualization in vitro, BMP2 mRNA increased during decidualization and addition of BMP2 to cultures accelerated PRL mRNA expression (a measure of the extent of decidualization). BMP2 protein has not been examined in vivo in human endometrial tissue. The only GDFs identified in the uterus are GDF9 and GDF10, detected by northern analysis in both human and mouse (Cunningham et al., 1995; Fitzpatrick et al., 1998; Zhao et al., 1999): no studies have shown GDF proteins.
TGFβ isoforms (1–3), which cannot bind follistatin, have also been localized in human endometrial stroma (Jones et al., 2006), although it is not clear whether any isoform is specific to decidualized cells or increases as the cells decidualize. The TGFβs transmit signal through the TGFβ type I, II and III receptors, all of which are present on endometrial stromal cells (Chegini et al., 1994; Dumont et al., 1995). Microarray studies have demonstrated increases in both TGFβ1 and TGFβ2 mRNA with HESC decidualization in vitro (Popovici et al., 2000; Tierney et al., 2003), while in women treated with medroxyprogesterone acetate (MPA) for 10 days, stromal mRNA and protein increased for TGFβ3 but not TGFβ1 (Reis et al., 2002). Whether the TGFβs play a functional role during human decidualization remains unknown.
Given that the TGFβ superfamily of proteins are such common and crucial differentiative and proliferative factors, it is surprising that very few members of the family, including the GDFs and BMPs, have been examined in the human endometrium, particularly in the mid-secretory phase when differentiation of stromal cells is initiated. The high level of redundancy between TGFβ family members and the promiscuity of receptor–ligand interactions would suggest that it is likely that a number of other family members are involved in decidualization and in the other processes occurring in this highly dynamic tissue.
In this study, the presence of BMPs (BMP2, BMP4, BMP7), GDFs (GDF5, GDF8/Myostatin, GDF11), Nodal and TGFβs were examined in vivo by immunohistochemistry in secretory phase endometrial tissue, and in vitro for mRNA expression in both non-decidualized and decidualized HESC. Inhibitors with different specificities were administered to HESC to elucidate whether activin is the major family member driving decidualization or whether other family groupings (BMPs, GDFs, TGFβs) might equally contribute to the process. As these are secreted factors, individual ligands were measured in conditioned medium from non-decidualized and decidualized HESC to assess whether they increased with decidualization. The effect of the secreted ligands on decidualization was also examined. It is important to identify which TGFβ members are important during decidualization since this process is critical for implantation and the establishment of pregnancy.
Materials and Methods
Tissue collections
Endometrial biopsies (n = 18) were collected by dilatation and curettage from fertile women who were scheduled for tubal ligation or were undergoing testing for tubal patency. Tissues were assessed by a pathologist and had no obvious endometrial pathology. The women had no steroid treatment or other medication for at least 2–3 months before the collection of tissue. Written and informed consent was obtained from all women participating in the study, and the protocols were approved by Monash Medical Centre Human Ethics Committee.
Immunohistochemistry
Immunohistochemical analysis was performed using a total of nine endometrial tissue biopsies from fertile women, confirmed by Noyes criteria (Noyes et al., 1975) as mid-late secretory phase tissue (POD 6–12). For each of the antibodies, activin βA and βB, BMP2, BMP4, BMP7, GDF5, GDF8, GDF11, Nodal and TGFβ1, n = 4–5 different tissue biopsies were used. For immunolocalization in first trimester placental tissue (kindly provided by Professor Euan Wallace, Obstetrics and Gynaecology, Monash University, Melbourne, Australia), n = 2 different biopsies were used per antibody. Briefly, 5 µm sections of formalin-fixed, paraffin-embedded tissues were dewaxed and rehydrated. For each antibody an antigen retrieval step was required, which involved microwave exposure or trypsin digestion (0.01% in CaCl2for 10 min at 37°C). Endogenous hydrogen peroxidase activity was quenched using 3% H2O2 in dH2O for 10 min at room temperature. Non-specific binding was prevented by pre-incubation of tissue sections with a non-immune block [5% fetal calf serum (FCS), 2% normal human serum in 0.1% Tween/Tris-buffered saline (TBS) with addition of 10% normal horse serum for BMP2, BMP4, BMP7, GDF5, GDF8; 10% normal swine serum for Nodal; normal goat serum for activin βA and βB, GDF11, TGFβ1]. Primary antibodies were against activin βA and activin βB (400 and 600 µg/ml, respectively; both gifts provided by W. Vale, Salk Institute, La Jolla, CA, USA); BMP2, BMP4, BMP7, GDF5, GDF8, Nodal (200 µg/ml; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA); GDF11 (1 mg/ml; Orbigen, San Diego, CA, USA); TGFβ1 (μg/ml; Abcam, Cambridge, UK). Specificity for these antibodies has been previously published (Nadiri et al., 2004; Kiyono and Shibuya, 2003; Mendler et al., 2000; McPherron et al., 1999; Garba and Relinger, 2001). For GDF5 and Nodal, the specificity has been shown by western blot analysis (unpublished observation). Antibodies were diluted to 2–4 µg/ml in the non-immune block and applied overnight (18–20 h) at 4°C. A non-immune goat/rabbit/mouse IgG (R&D Systems Incorporated, Minneapolis, MN, USA) was used at a matching concentration to the primary antibody and was included for each tissue. After stringent washing with high salt TBS and 0.6% Tween 20 (BioRad Laboratories, Hercules, CA, USA), detection of positive binding was performed by the sequential application of the appropriate secondary antibody diluted in non-immune block and avidin–biotin–peroxidase conjugate (Dako, Glostrup, Denmark). This was followed by the substrate diaminobenzidine (Dako) for between 3 and 5 min. Sections were counterstained with Harris' hematoxylin (Sigma Chemical Company, St Louis, MO, USA), dehydrated, and mounted from Histosol with DPX mounting medium (BDH Laboratory Supplies, Poole, UK). Immunostaining was analysed by two independent observers for staining intensity and heterogeneity in each of the endometrial compartments (glandular and luminal epithelium; stroma, including decidualized stromal cells; vasculature).
Stromal cell isolation and culture
Tissue biopsies ranging from Days 10–21 were used for in vitro decidualization studies. HESC were isolated from tissue by enzymatic digestion and filtration as described previously (Dimitriadis et al., 2002). Briefly, tissue was finely minced with scissors and digested by bacterial collagenase type III (Worthington Biochemical Corporation, Freehold, NJ, USA) at a concentration of 45 IU/ml, in the presence of 3.5 µg/ml deoxyribonuclease (DNase; Boehringer Mannheim Biochemica, Mannheim, Germany). After 30–45 min of agitation at 37°C, the digested tissue was filtered sequentially through 45 and 10 µm nylon filters to remove glands. Cell suspensions were centrifuged at 1500 rpm for 5 min. Cells were plated at a density of 5 × 106 in a 75 cm2 flask and grown to confluency in DMEM and Ham's F12 medium (1:1) (DMEM/F12) (Trace Biosciences, Sydney, Australia), supplemented with 1% PSF (penicillin, streptomycin and fungizone) (Commonwealth Serum Laboratories, Melbourne, Australia) and 10% charcoal stripped FCS (csFCS) (Thermo Scientific, Maple Plain, USA). Approximately 30 min after plating, medium containing non-attached epithelial cells was removed and replaced with fresh medium. This results in a >97% pure stromal cell culture (Dimitriadis et al., 2002).
In vitro decidualization
Confluent HESC were rinsed with phosphate-buffered saline, trypsinized and replated into 24-well plates using DMEM/F12 and 10% csFCS. Once confluent, HESC were washed with DMEM/F12 and the medium replaced with a serum-free medium containing DMEM/F12 and a serum-free mix (SFM) including transferrin (10 µg/ml; Sigma), sodium selenite (25 ng/ml; Sigma), linoleic acid (10 nmol/L; Sigma), bovine serum albumin (0.1%; Sigma) and insulin (5 µg/ml; Actrapid, Novo-Nordisk Pharmaceuticals Pty Ltd, Sydney, Australia) for 48 h prior to treatment addition. HESC were decidualized by two distinct methods as previously described (Dimitriadis et al., 2005). For the inhibitor, mRNA expression and protein secretion studies, 0.5 mM cAMP (Sigma) was added to medium for 4–6 days. Alternatively, when exogenous proteins were added, cells were decidualized with E2 (10−8 M; Sigma) and MPA (10−7M; Sigma) for 8 days. All media were replaced every 48–72 h.
RNA extraction and cDNA synthesis
Total RNA was extracted from non-decidualized and decidualized HESC from two separate biopsies at Day 4–6 using the RNeasy Minikit (Qiagen Sciences, Germantown, MD, USA), according to the manufacturer's instructions. RNA from positive control tissues was extracted by homogenization in Trizol reagent (Qiagen Sciences, Clifton Hill, Victoria, Australia), according to the manufacturer's instructions, with the exception of an additional chloroform extraction step to minimize carryover of phenol into the precipitate. The control tissues used were: term placenta (activin βA, βB, GDF11, TGFβ1, -2, -3); pregnant mouse endometrium (BMP2); mature rat ovary (BMP4, GDF5); cycling mouse endometrium (BMP7); mouse heart (GDF8); JEG3 cells (Nodal). All samples were treated with RNase-free DNase (Ambion, Austin, TX, USA) to remove the possibility of genomic DNA contamination. RNA samples were then analysed by spectrophotometry to determine RNA concentration, yield and purity. Total RNA (1 µg) was reverse transcribed at 46°C for 1.5 h in 20 µl reaction mixture using 100 ng random hexanucleotide primers and 6 IU AMV reverse transcriptase (Roche, Castle Hill, Australia) in the presence of cDNA synthesis buffer (Roche), 1 mmol/l dNTPs (Roche), 10 mmol/l dithiothreitol (Roche), 10 IU ribonuclease inhibitor (RNasin; Promega, Annandale, Australia). The resultant cDNA mixtures were heated at 95°C for 3 min before storage at −20°C. Negative controls were performed by omission of reverse transcriptase. Triplicate RNA samples were reverse transcribed in triplicate for each condition (non-decidualized and decidualized) with the successful conversion to cDNA monitored by 18S expression (data not shown).
RT–PCR
Messenger RNA expression for all named TGFβ superfamily ligand members was determined using a conventional PCR block cycler (Hybaid, Middlesex, UK). All ligands (excluding Nodal), used a 1 µl aliquot of RT product, to be amplified in a total volume of 40 µl using 4 µl of RT single strength PCR buffer (10 mmol/l Tris–HCl, 1.5 mmol/l MgCl2, 50 mmol/l KCl, pH 8.3; Roche), 2.5 mmol/l dNTPs (Gibco, Melbourne, Australia), 0.5 pmol/µl sense and antisense primers (Sigma Genosys Australia Pty Ltd, Castle Hill, Australia) and 2.5 IU Taq DNA polymerase (Roche). For Nodal, 1 µl of RT was amplified in a total of 50 µl using the KOD-Taq PCR kit (Bioron, Germany), which included 10× PCR KOD Hot Start buffer, 2 mM dNTPs, 0.5 pmol/μl primers, 2 mM MgSO4 and 2.5 IU Taq DNA polymerase (Roche). For all ligands, the PCR was performed in three stages as follows: the first stage involved 94°C for 5 min, x C for 1 min, where x is the annealing temperature for the individual primer pairs (see Supplementary data) and 72°C for 3 min; the second stage involved 35–40 cycles of 94°C for 1 min, x C for 1 min, and 72°C for 1 min; and the final stage was 72°C for 7 min. PCR products including positive controls were analysed by electrophoresis on a 2% agarose gel (Roche) and stained with ethidium bromide. Bands of interest were excised from the gel, purified (DNA purification kit, Qiagen) and directly sequenced to confirm their identity.
Inhibitor experiments
For examination of the effect of inhibitors on decidualization, cells (from n = 10 biopsies) were plated at a density of 2.5 × 105/well in 24-well plates until confluent, and then equilibrated in serum-free medium containing SFM for 48 h. This was designated Day 0 and first day of treatments. HESC were exposed to varying doses of either the inhibitor Activin-M108A (M108A) (0.39 1.56, 6.25 and 25 nM) (Harrison et al., 2004, 2006) or the inhibitor SB431542 (1.25, 2.5, 5 and 10 µM) (TOCRIS Bioscience, Northpoint, UK). Following 1 h incubation with either inhibitor, 0.5 mM cAMP (Sigma) was added as decidualizing stimulus. Each treatment was performed in triplicate wells of a 24-well plate and the experiments ran for 4–6 days with media collection and replenishment (including inhibitors) every 48–72 h. In each case, the final medium collection represented the final 48 h. Each experiment included a medium-only and a cAMP-only control. Cells were photographed for morphological analysis and cells from triplicate wells were pooled for trypan blue cell exclusion to check cell viability at the end of each experiment. Five separate cultures were conducted for each inhibitor.
TGFβ superfamily assays
Culture medium from triplicate wells from three tissue biopsies (n = 3 separate decidualization experiments) were collected, pooled and concentrated 5-fold to measure BMP2 (ELISA; R&D Systems), BMP4, BMP7, TGFβ1 and TGFβ2 (ELISA; Ray BioTech., Norcross, GA, USA) according to the manufacturer's instructions. Mean sensitivities of the assays were: BMP4, 15 pg/ml, BMP7, 10 pg/ml, TGFβ1, 80 pg/ml, TGFβ2, 15 pg/ml, with intra-assay CV <10% and inter-assay CV <12% for each assay. Mean sensitivity for BMP2 was 11 pg/ml and intra- and inter-assay variabilities were 2.6 and 6.3%, respectively. TGFβ1 and TGFβ2 ELISAs measured only the activated protein form and required activation steps prior to assay. Dimeric activin A secretion from stromal cells was measured by Activin A Immunofluorometric Assay (IFMA) (Harrison et al., 2006). The working range of the assay is 0.03–3 ng/well, with a sensitivity of 0.03 ng/well. All assays were read at 450 nm.
Addition of exogenous BMP2 and TGFβ1 during decidualization
HESC from three separate cultures were decidualized using E2 and MPA as described above, in the presence of varying doses of either recombinant human (rh) BMP2 (5, 50, 500 ng/ml; R&D Systems) or TGFβ1 0.5, 5, 50 ng/ml; PeproTech Inc., NJ, USA) added every 48 h with media and decidualizing stimulus replenishment. At Day 8, cells were morphologically assessed and conditioned media were collected for PRL measurement.
PRL and protein assays
PRL production by HESC was assayed in duplicate by ELISA (Bioclone Australia Pty Ltd, Sydney, Australia) to determine the extent of decidualization (Dimitriadis et al., 2002). Media collected at the end of the experiment were concentrated 5-fold for PRL measurement. A quality control sample (culture medium from a single endometrial cell culture) was included in every assay. The lower detection limit of the assay was 50 mIU/l. The inter- and intra-assay variablilities were 5.3 and 3.0%, respectively. PRL concentrations (mIU/l) were corrected for the amount of protein (μg/μl) determined using the Bradford reagent.
Statistical analysis
Data were expressed as mean ± SEM. Statistical significance for inhibitor studies was determined following confirmation of normal distribution, by one-way ANOVA followed by Dunnett's multiple comparisons test. Data from ELISA and recombinant protein studies were analysed by Student's t-test. A P-value of <0.05 was considered statistically significant.
Results
Localization of TGFβ superfamily ligands in human endometrium and first trimester decidua
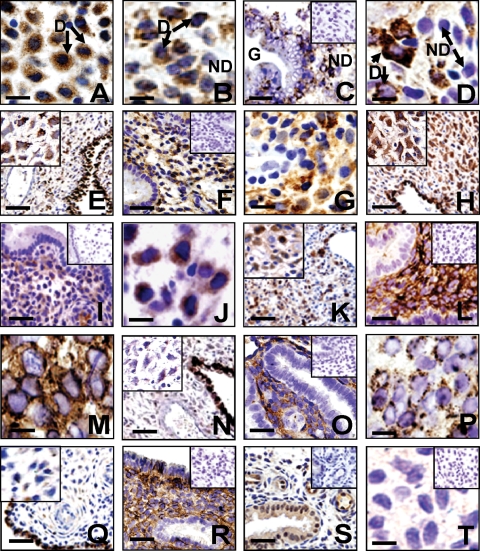
All TGFβ superfamily members studied, except Nodal, were detected in mid-late secretory endometrium and first trimester decidua. As previously described (Jones et al., 2000), activin A and activin B were localized to the morphologically distinct decidual cells (Fig. 1A and B), glandular and luminal epithelial cells and some leukocytes (data not shown). Some glands had punctate staining for BMP2 (Fig. 1C), but BMP2 was most strongly stained in decidual cells in mid-late secretory endometrium (Fig. 1D). In first trimester decidua, BMP2 protein was detected in both the decidual cells (Fig. 1E, inset) and glandular epithelium, while the vessels were devoid of stain (Fig. 1E). GDF5 protein was observed in the decidualized stromal cells (Fig. 1F and G) and was very low in the glands (Fig. 1F) and luminal epithelium (data not shown). Similarly, GDF5 was very strongly expressed by the decidua of first trimester placenta, as well as the glandular epithelium (Fig. 1H, inset). TGFβ1 was present in decidualized stromal cells (Fig. 1I and J), with minimal staining evident in the glandular epithelium (Fig. 1I). As previously described (Graham et al., 1992), TGFβ1 was detected in decidual cells and extravillous trophoblasts of first trimester placenta (Fig. 1K). BMP4 was not detected in the glandular or luminal epithelium, but was present in the cytoplasm of both non-decidualized and decidualized stromal cells (Fig. 1L and M). However, in first trimester placenta, BMP4 was reduced in the decidual cells, but strongly produced by the glandular epithelium (Fig. 1N, inset). BMP7 was detected in the stroma and not in the glands in mid-late secretory endometrium (Fig. 1O). Stromal staining for BMP7 did not appear to be decidual cell specific, however, in highly decidualized tissue stronger ‘vesicle staining’ was evident (Fig. 1P). Similar but faint punctate staining for BMP7 was detected around spiral arterioles of first trimester placenta, but not specifically in decidual cells (Fig. 1Q, inset). BMP7 was also strongly localized to glandular and luminal epithelium (Fig. 1Q). GDF8 immunostaining in mid-late secretory endometrium showed minimal glandular and luminal epithelial staining, and intense stromal cell cytoplasmic staining, in both non-decidualized and decidualized cells (Fig. 1R). GDF11 immunolocalized to glandular epithelium and endothelial cells, but was very low in the stroma (Fig. 1S). GDF8 and GDF11 in first trimester placenta showed similar staining patterns to BMP4 (data not shown). No immunoreactive Nodal was detected in late secretory endometrium (Fig. 1T), although the immunostaining protocol was verified by positive staining in term placenta (not shown).
Figure 1:
Localization of TGFβ superfamily members during decidualization in mid-late secretory endometrium and first trimester decidua.
Photomicrographs are representatives of immunostaining for activin A (A), activin B (B), BMP2 (C–E), GDF5 (F–H), TGFβ1 (I–K), BMP4 (L–N), BMP7 (O–Q), GDF8 (R), GDF11 (S), Nodal (T). In mid-late secretory endometrium; scale bar = 100 µm (C, F, I, L, O, R, S); scale bar = 10 µm (D, G, J, M, P, T). In first trimester decidua; scale bar = 200 µm (E, H, K, N, Q); inserts show higher power images of same tissue (E, H, K, N, Q). Negative controls for each antibody are shown in inserts (C, F, I, L, O, R, S, T). Arrows highlight decidualized (D), non-decidualized (ND) stromal cells and glands (G).
TGFβ superfamily mRNA expression in non-decidualized and decidualized HESC
By RT–PCR, mRNA expression was evident for activin βA, activin βB, BMP2, BMP4, BMP7, GDF5, GDF8, GDF11, TGFβ1, TGFβ2 and TGFβ3 in both non-decidualized and decidualized HESC (Fig. 2). Nodal mRNA was undetectable in both. As conventional RT–PCR was used, only the presence/absence of each gene was assessed. Positive control PCR products (Fig. 2; see supplementary data) confirmed sequence identity and no signals were detected when reverse transcriptase was omitted (Fig. 2).
Figure 2:
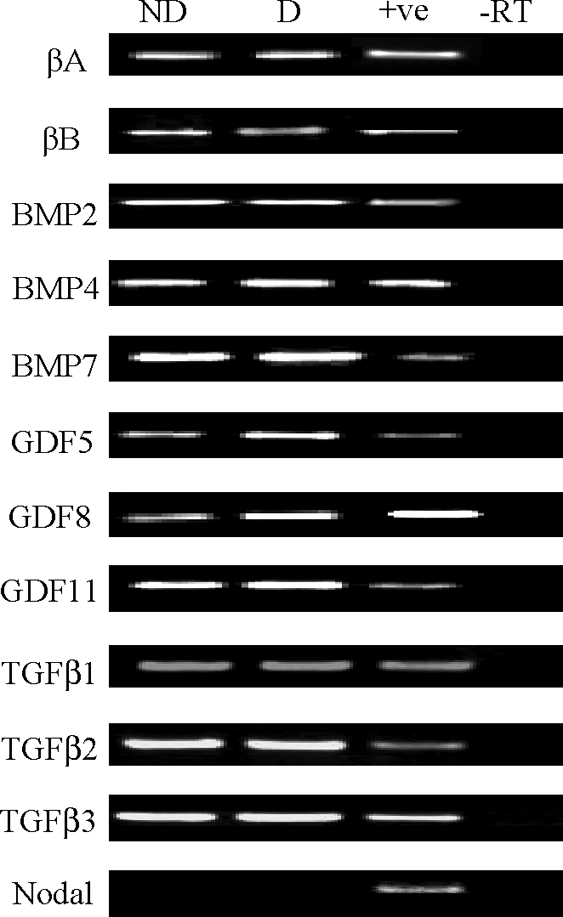
mRNA expression for TGFβ superfamily members by decidualized and non-decidualized HESC.
HESC were either non-decidualized or decidualized with cAMP for 7 days. Total RNA was extracted from cells for RT–PCR. Representative products demonstrating Activin βA, Activin βB, BMP2, BMP4, BMP7, GDF5, GDF8, GDF11, TGFβ1, TGFβ2, TGFβ3 and Nodal mRNA are shown in non-decidualized (ND) and cAMP-decidualized (D) HESC; +ve=positive control; −RT=negative control. Data shown is from a single experiment, which is representative of 2 independent culture experiments.
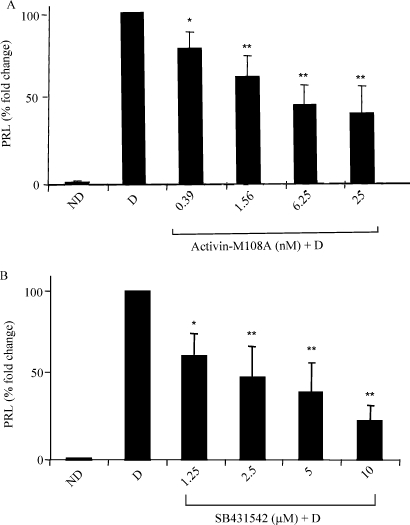
Activin-M108A and SB431542 significantly decreases PRL secretion during HESC decidualization
To establish whether HESC decidualization could be blocked or reduced, the newly developed activin type II receptor antagonist, Activin-M108A or the type I receptor kinase inhibitor, SB431542 were used in an in vitro decidualization model with PRL secretion, a marker of decidualization, as the end-point. These inhibitors affect activin and various other TGFβ family members (see Table I). Cells maintained in medium alone (non-decidualized) showed non-detectable PRL levels over the final 48 h of culture (Fig. 3). In contrast, cells treated with cAMP secreted PRL (mean 372.6 ± 145.5 mIU/l), and morphologically changed from elongated spindle-shaped cells to typical enlarged polygonal cells demonstrating successful decidualization, as previously described (Dimitriadis et al., 2002). Stromal cells cultured with cAMP and Activin-M108A showed a significant dose-dependent decrease in PRL secretion at 0.39, 1.56 and 6.25 nM with no further decrease at 25 nM (Fig. 3A). At the 25 nM dose, a 60% reduction in PRL secretion was seen compared with cAMP-treated cells without inhibitor, which was similar to decidualization reduction seen with follistatin. Similarly cells co-cultured with cAMP and SB431542 showed a dose-dependent decrease in PRL secretion. Addition of SB431542 at a concentration of 1.25 µM significantly reduced PRL secretion compared with cAMP-only treated cells (Fig. 3B). Further significant decreases were observed with doses ranging from 2.5–10 µM (Fig. 3B). At 10 µM, SB431542 reduced PRL secretion by 77% compared with cAMP alone (Fig. 3B). Higher doses were not tested. When either inhibitor (Activin-M108A or SB431542) was administered to cells in the absence of cAMP, PRL was not detected in culture medium. In addition, cell viability was tested using the trypan blue exclusion method at the end of the experiment and was found to not differ between treatments with medium-only, cAMP and either inhibitor. Microscopic visualization indicated cell morphology was not altered by inhibitor use, suggesting this effect was not due to toxicity to cells.
Figure 3:
Activin-M108A and SB43 154 inhibitors significantly decrease HESC decidualization.
Confluent stromal cells were cultured for 4–5 days with cAMP, with medium changes every 2–3 days. (A) M108A was added at doses 0.39, 1.5, 6.25 and 25 nM to cAMP-treated cultures. (B) SB431542 was added at doses 1.25, 2.5, 5 and 10 µM. Controls (not decidualized; ND) and cAMP (decidualized; D). PRL secretion from cells was used as a marker of decidualization, and values were corrected for total protein. All data (mean ± SEM) is expressed as % change from control (100%). Results are combined data from triplicate wells of five independent experiments for each inhibitor. *P < 0.05 and **P < 0.01 compared with cAMP alone.
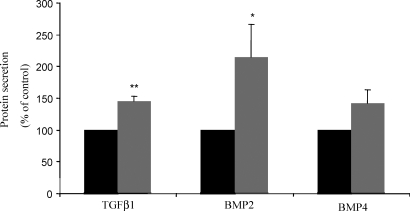
Secretion of activin A, TGFβ1, BMP2 and BMP4, but not TGFβ2 or BMP7 from non-decidualized and decidualized HESC in vitro
TGFβ ligands are active as secreted proteins. Therefore we measured BMP2, BMP4, BMP7, TGFβ1, TGFβ2 from three different cultures by ELISA and activin A by IFMA (n = 1) in serum-free culture medium. There was a 4-fold increase in dimeric activin A secreted from decidualized (1.9 ng/106cells) compared with non-decidualized cells (0.43 ng/106cells), supporting previous published data from our laboratory (Jones et al., 2002). Non-decidualized and decidualized HESC secreted BMP2, TGFβ1 and BMP4, each in the range of 5–20 pg/106 cells (Fig. 4). BMP7 and TGFβ2 levels were below the sensitivity of the assays (data not shown). Decidualized stromal cells secreted significantly higher amounts of BMP2 and TGFβ1 compared with non-decidualized cells (TGFβ1 20.6 ± 3.8 versus 14 ± 3.1 pg/106cells; BMP2 17.2 ± 2.32 versus 8.44 ± 1.5 pg/106cells) (Fig. 4). BMP4 secretion was increased slightly with decidualization (6.9 ± 2.17 versus 10.1 ± 3.45 pg/106cells), but this was not significant (Fig. 4). Western blot analysis was performed for GDF5, GDF8, GDF11 and TGFβ3 using 100–150 µg of cell lysates from non-decidualized and decidualized HESC; however, these proteins were below the level of detection. Positive controls (GDF5—rat heart and human term placenta; TGFβ3—human term placenta; GDF8 and GDF11—recombinant human myostatin/GDF8 and recombinant human GDF11, respectively; data not shown) validated the technique.
Figure 4:
BMP2, TGFβ1 and BMP4 protein secretion by HESC during decidualization.
BMP2, BMP4 and TGFβ1 protein were measured in culture medium from non-decidualized (dark) and decidualized (grey) cells at Day 4 of culture. Results are combined data from n = 3 independent culture experiments and data (mean ± SEM) is represented as % difference from control (non-decidualized cells; defined as 100%). *P < 0.05 and **P < 0.01 compared to control.
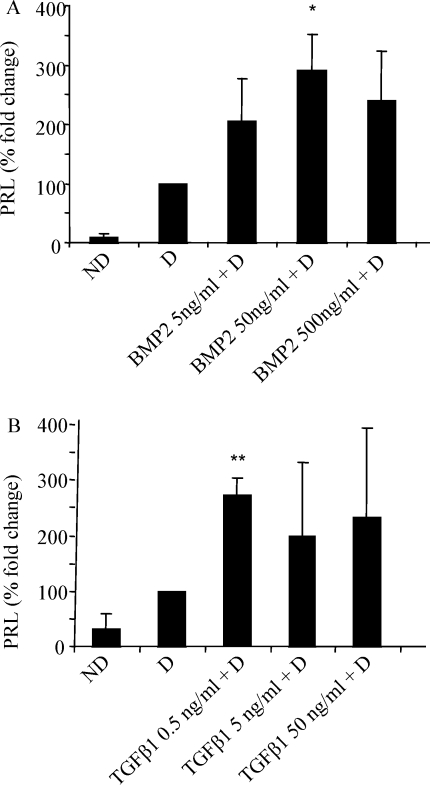
Exogenous BMP2 and TGFβ1 increases HESC decidualization in vitro
To assess whether addition of rhBMP2 and/or rhTGFβ1 to HESC enhances decidualization in vitro, cells were decidualized with E2 and MPA under serum-free conditions for 8 days and PRL secretion measured. At Day 8, PRL secretion from non-decidualized cells was minimal but was increased when cells were decidualized (Fig. 5). When rhBMP2 (5, 50 or 500 ng/ml) was added to decidualizing HESC, PRL secretion was significantly increased at the 50 ng/ml dose compared with the decidualized control (P < 0.05). The addition of rhTGFβ1 at 0.5, 5 or 50 ng/ml to decidualizing HESC, however this was only significant at the 0.5 ng/ml dose (P < 0.01) compared with decidualized cells alone.
Figure 5:
Effect of exogenous BMP2 and TGFβ1 on PRL secretion from HESC.
Cells were untreated or treated with E2 (10–8 M), MPA (10–7 M) alone or combined with recombinant human (rh) BMP2 (5–500 ng/ml) (A), rhTGFβ1 (0.5–50 ng/ml) (B) for 8 day. PRL was measured in cultured medium from the last 48 h of treatment. Data are represented as percent fold change from control (decidualized; D) defined as 100%. ND, non-decidualized HESC. Results are combined data from triplicate wells of three independent cultures. *P < 0.05 and **P < 0.01 compared with D.
Due to the variability of endometrial biopsies only one dose for each BMP2 and TGFβ1 has resulted in a significant promotion of decidualisation. Also as the rhTGFβ1 is known to be quite potent, this result could be explained by the fact that at the higher doses it might be negatively affecting the response, for example receptor and downstream signalling disturbances.
Discussion
This is the first study to identify BMP2, BMP4, BMP7, GDF5, GDF8 and GDF11 protein in secretory phase human endometrium and show the mRNA expression of these ligands in cultured HESC. In addition, the current study has immunolocalized BMP2, TGFβ1 and GDF5 protein to decidualized stromal cells, and provided further evidence for a role for secreted activin, BMP2 and TGFβ1 in decidualization through the use of novel inhibitors and a well-established ex vivo decidualization model.
Decidualization of endometrial stromal cells is a pivotal event in the preparation for blastocyst implantation. Although this process is governed by hormonal regulation, it is becomingly increasingly evident that a multitude of factors, including cytokines and growth factors, are essential in ensuring this process occurs successfully such that endometrial stromal cells are differentiated into the implantation-favourable decidual cells. The identification of BMP2, TGFβ1 and GDF5 protein in mid-secretory human endometrial tissue, together with the increased secretion of both BMP2 and TGFβ1 from stromal cells following decidualization, suggests these TGFβ family members are hormonally regulated and important for decidualization.
The finding that BMP2 secretion from HESC increases as they decidualize in vitro and that addition of rhBMP2 further drives decidualization as determined by secretion of PRL, extends the recent study (Li et al., 2007), which showed an increase of BMP2 mRNA with decidualization in a similar model and of PRL mRNA following treatment of HESC with rhBMP2. This is important given that translation and secretion do not necessarily follow increases in mRNA. The inhibitor M108A which acts primarily on those ligands acting through the activin type 11 receptor (Table I) is not known to block the action of BMP2 which acts through the BMP type II receptor (BMPRII). Therefore, the inability of M108A to fully block the decidualization process suggests that factors other than activins and GDFs are likely to be important to this process: BMP2 is a likely candidate.
Interestingly in mice, uterine-specific conditional ablation of BMP2 resulted in uterine stromal disruption and an inability to undergo normal decidual reactions and pregnancy progression (Lee et al., 2007). Our demonstration of BMP2 in human endometrium in vivo with the strongest immunostaining in decidualized cells in the mid-late secretory phase and in the decidua of early pregnancy, confirms the biological relevance in women. Given this data in conjunction with our functional studies of human decidualization, there appears to be little doubt that BMP2 is an important mediator of both mouse and human decidualization.
This study also demonstrates, for the first time, the presence of GDF5 in human endometrium and first trimester placenta where it is intensely and specifically stained in the decidual cells. GDF5 is best known for its role in early chondrogenesis and joint formation, being involved in inducing cartilage differentiation, growth and maturation (Mikic, 2004), as well as bone formation and angiogenesis (Yamashita et al., 1997). Differentiation and angiogenesis are the main events of decidualization. Unfortunately, assays for GDF5 were not available to measure the secretion from decidualized cells. The decrease in decidualization seen following administration of M108A was not likely to be due to GDF5 blockade, as this ligand has a relatively low, if any, affinity for the activin type II receptor. Future studies should address whether GDF5 has a functional role in decidualization, whether it be in promoting differentiation or in the formation of associated blood vessels to support the blastocyst in early pregnancy and throughout gestation.
Although the TGFβs have been previously detected in the human endometrium, the information regarding their role during decidualization is contentious, as studies report contrasting effects on PRL production by endometrial stroma and during decidualization in vitro (Kubota et al., 1997; Kim et al., 2005). We showed here that under serum-free conditions, TGFβ1 secretion increases during cAMP-induced decidualization in vitro. Microarray studies have likewise shown increased TGFβ1 and TGFβ2 mRNA when cAMP or progesterone is used as decidual stimuli (Popovici et al., 2000; Tierney et al., 2003). The protein for both isoforms localizes to stromal cells in human endometrium throughout the menstrual cycle (Gold et al., 1994; Godkin and Dore, 1998), although decidualized stromal cells were not shown. In contrast, the present study identified TGFβ1 protein in decidualized stromal cells in human endometrium, and its secretion increased in cAMP-decidualized HESC. Administration of SB431542, which effectively blocks the TGFβs as well as activins by blocking their structurally similar ALK5 and ALK4 receptors (Inman et al., 2002), resulted in a 77% reduction in decidualization, a greater decrease than that seen with M108A (this study) and follistatin (Tierney and Giudice, 2004). This, along with our demonstration that addition of TGFβ1 during progesterone-induced decidualization enhances decidualization, provides convincing evidence to suggest TGFβ1 is important during decidualization. The level of activated TGFβ2 was undetectable by ELISA in cAMP-decidualized endometrial stromal cells, and therefore this ligand is unlikely to contribute to the decidual response.
The identification of BMP4, BMP7, GDF8 and GDF11 during the mid-secretory phase is also an important finding. In addition to decidualization, there are many remodelling processes occurring in the endometrium during this time. Both BMP4 and GDF8 protein showed intense immunolocalization to the cytoplasm of the stroma, irrespective of whether or not the cells were decidualized. This was verified for BMP4 by the minimal difference in secretion levels between non-decidualized and decidualized cells in vitro. BMP4 mRNA expression has been localized to the vascular endothelial cells in pregnant mice (Ying and Zhao, 2000), and in the non-pregnant rat uterus, where it is constitutively expressed throughout the cycle (Erickson et al., 2004). This differs to the localization of BMP4 protein shown here in human endometrium. It may be that the mRNA localized to blood vessels in the rodent studies is not translated into functional protein.
GDF8 (more commonly referred to as Myostatin) is a negative regulator of skeletal muscle, but more recently has been shown to be involved in glucose metabolism (McPherron and Lee, 2002), particularly in placenta (Mitchell et al., 2006). This study verified the presence of GDF8 protein in first trimester placenta, and showed for the first time its presence in secretory phase endometrium and in HESC. Given the lack of specific decidual staining it is unlikely that the response from M108A inhibitor was due to blocking GDF8 in HESC, even though the predominant receptor for GDF8 is the activin type II receptor. The presence of GDF8 in non-muscular tissue and organs is rare and future experiments should determine whether it has a role in endometrial biology as it does throughout gestation in the placenta.
Vesicular staining for BMP7 (or Osteogenic protein-1; OP-1) was evident in highly decidualized cells during the secretory phase of human endometrium with minimal staining in selected glandular and luminal epithelial cells. OP-1 has been identified in the uterine epithelium of non-pregnant mice and rat uterus (Ozkaynak et al., 1997; Erickson et al., 2004), with localization remaining unchanged across the cycle. In pregnant mice, however, BMP7 mRNA was detected in the decidualizing stromal cells surrounding the blastocyst with no staining in the epithelial cells (Ying and Zhao, 2000). In the present study, BMP7 secretion from HESC was undetectable by ELISA, making it difficult to assess whether it may have a functional role during decidualization.
In conclusion, this study has identified that a wide range of TGFβ ligands are present in mid-late secretory human endometrium but of these, in addition to activins, only BMP2, GDF5 and TGFβ1 increase in stromal cells as they undergo decidualization. BMP2 and TGFβ1, along with activins, are important in driving the decidualization process. Functions for the remaining ligands remain to be determined but they are likely to participate in the extensive tissue remodelling that occurs in this highly dynamic tissue as it prepares for blastocyst implantation and pregnancy.
Supplementary material
Supplementary material is available at HUMREP Journal online
Funding
This work was funded by the National Health and Medical Research Council of Australia (#241000, #388901) and a Prince Henry's Institute Postgraduate Scholarship.
Supplementary Material
Acknowledgements
We thank Professor E. Wallace (Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia) for providing human placental tissue and Karen Chan for technical assistance.
References
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Bell SC. The insulin-like growth factor binding proteins—the endometrium and decidua. Ann N Y Acad Sci. 1991;622:120–137. doi: 10.1111/j.1749-6632.1991.tb37856.x. [DOI] [PubMed] [Google Scholar]
- Chegini N, Gold LI, Williams RS. Localization of transforming growth factor beta isoforms TGF-beta 1, TGF-beta 2, and TGF-beta 3 in surgically induced endometriosis in the rat. Obstet Gynecol. 1994;83:455–461. [PubMed] [Google Scholar]
- Cunningham NS, Jenkins NA, Gilbert DJ, Copeland NG, Reddi AH, Lee SJ. Growth/differentiation factor-10: a new member of the transforming growth factor-beta superfamily related to bone morphogenetic protein-3. Growth Factors. 1995;12:99–109. doi: 10.3109/08977199509028956. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Stoikos C, Baca M, Fairlie WD, McCoubrie JE, Salamonsen LA. Relaxin and prostaglandin E(2) regulate interleukin 11 during human endometrial stromal cell decidualization. J Clin Endocrinol Metab. 2005;90:3458–3465. doi: 10.1210/jc.2004-1014. [DOI] [PubMed] [Google Scholar]
- Dumont N, O'Connor-McCourt MD, Philip A. Transforming growth factor-beta receptors on human endometrial cells: identification of the type I, II, and III receptors and glycosyl-phosphatidylinositol anchored TGF-beta binding proteins. Mol Cell Endocrinol. 1995;111:57–66. doi: 10.1016/0303-7207(95)03548-l. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Fuqua L, Shimasaki S. Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle. J Endocrinol. 2004;182:203–217. doi: 10.1677/joe.0.1820203. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Sindoni DM, Shughrue PJ, Lane MV, Merchenthaler IJ, Frail DE. Expression of growth differentiation factor-9 messenger ribonucleic acid in ovarian and nonovarian rodent and human tissues. Endocrinology. 1998;139:2571–2578. doi: 10.1210/endo.139.5.6014. [DOI] [PubMed] [Google Scholar]
- Frank GR, Brar AK, Cedars MI, Handwerger S. Prostaglandin E2 enhances human endometrial stromal cell differentiation. Endocrinology. 1994;134:258–263. doi: 10.1210/endo.134.1.7506205. [DOI] [PubMed] [Google Scholar]
- Garba ML, Relinger JA. Intracellular cytokine staining for TGf-beta. J Immunol Methods. 2001;258:193–8. doi: 10.1016/s0022-1759(01)00491-4. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev Biol. 1999;208:222–232. doi: 10.1006/dbio.1998.9191. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- Glister C, Kemp CF, Knight PG. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction. 2004;127:239–254. doi: 10.1530/rep.1.00090. [DOI] [PubMed] [Google Scholar]
- Godkin JD, Dore JJ. Transforming growth factor beta and the endometrium. Rev Reprod. 1998;3:1–6. doi: 10.1530/ror.0.0030001. [DOI] [PubMed] [Google Scholar]
- Gold LI, Saxena B, Mittal KR, Marmor M, Goswami S, Nactigal L, Korc M, Demopoulos RI. Increased expression of transforming growth factor beta isoforms and basic fibroblast growth factor in complex hyperplasia and adenocarcinoma of the endometrium: evidence for paracrine and autocrine action. Cancer Res. 1994;54:2347–2358. [PubMed] [Google Scholar]
- Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-beta at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod. 1992;46:561–572. doi: 10.1095/biolreprod46.4.561. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Fischer WH, Donaldson C, Choe S, Vale W. An activin mutant with disrupted ALK4 binding blocks signaling via type II receptors. J Biol Chem. 2004;279:28036–28044. doi: 10.1074/jbc.M402782200. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Chan KL, Robertson DM. Activin-A binds follistatin and type II receptors through overlapping binding sites: generation of mutants with isolated binding activities. Endocrinology. 2006;147:2744–2753. doi: 10.1210/en.2006-0131. [DOI] [PubMed] [Google Scholar]
- Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N. Direct binding of follistatin to a complex of bone- morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci USA. 1998;95:9337–9342. doi: 10.1073/pnas.95.16.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Jones RL, Salamonsen LA, Critchley HO, Rogers PA, Affandi B, Findlay JK. Inhibin and activin subunits are differentially expressed in endometrial cells and leukocytes during the menstrual cycle, in early pregnancy and in women using progestin-only contraception. Mol Hum Reprod. 2000;6:1107–1117. doi: 10.1093/molehr/6.12.1107. [DOI] [PubMed] [Google Scholar]
- Jones RL, Salamonsen LA, Findlay JK. Activin A promotes human endometrial stromal cell decidualization in vitro. J Clin Endocrinol Metab. 2002;87:4001–4004. doi: 10.1210/jcem.87.8.8880. [DOI] [PubMed] [Google Scholar]
- Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132:217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- Kim MR, Park DW, Lee JH, Choi DS, Hwang KJ, Ryu HS, Min CK. Progesterone-dependent release of transforming growth factor-beta1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol Hum Reprod. 2005;11:801–808. doi: 10.1093/molehr/gah240. [DOI] [PubMed] [Google Scholar]
- Kiyono M, Shibuya M. Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression. Mol Cell Biol. 2003;23:4627–4636. doi: 10.1128/MCB.23.13.4627-4636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Taguchi M, Kobayashi K, Masuda M, Aso T. Relationship between the release of prolactin and endothelin-1 in human decidualized endometrial cells. Eur J Endocrinol. 1997;137:200–204. doi: 10.1530/eje.0.1370200. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt 4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007 doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- Loke YW, King A, Burrows TD. Decidua in human implantation. Hum Reprod. 1995;10(Suppl 2):14–21. doi: 10.1093/humrep/10.suppl_2.14. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nature Genetics. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler L, Zádor E, Ver Heyen M, Dux L, Wuytack F. Myostatin levels in regenerating trat muscles and in myogenic cell cultures. J Muscle Res Cell Motil. 2000;6:551–63. doi: 10.1023/a:1026542303629. [DOI] [PubMed] [Google Scholar]
- Mikic B. Multiple effects of GDF-5 deficiency on skeletal tissues: implications for therapeutic bioengineering. Ann Biomed Eng. 2004;32:466–476. doi: 10.1023/b:abme.0000017549.57126.51. [DOI] [PubMed] [Google Scholar]
- Mitchell MD, Osepchook CC, Leung KC, McMahon CD, Bass JJ. Myostatin is a human placental product that regulates glucose uptake. J Clin Endocrinol Metab. 2006;91:1434–1437. doi: 10.1210/jc.2005-2361. [DOI] [PubMed] [Google Scholar]
- Mylonas I, Jeschke U, Wiest I, Hoeing A, Vogl J, Shabani N, Kuhn C, Schulze S, Kupka MS, Friese K. Inhibin/activin subunits alpha, beta-A and beta-B are differentially expressed in normal human endometrium throughout the menstrual cycle. Histochem Cell Biol. 2004;122:461–471. doi: 10.1007/s00418-004-0709-6. [DOI] [PubMed] [Google Scholar]
- Nadiri A, Kuchler-Bopp S, Haikel Y, Lesot H. Immunolocalization of BMP-2/-4, FGF-4, and WNT10b in the developing mouse first lower molar. J Histochem Cytochem. 2004;52:103–12. doi: 10.1177/002215540405200110. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Otani T, Minami S, Kokawa K, Shikone T, Yamoto M, Nakano R. Immunohistochemical localization of activin A in human endometrial tissues during the menstrual cycle and in early pregnancy. Obstet Gynecol. 1998;91:685–692. doi: 10.1016/s0029-7844(98)00053-2. [DOI] [PubMed] [Google Scholar]
- Ozkaynak E, Jin DF, Jelic M, Vukicevic S, Oppermann H. Osteogenic protein-1 mRNA in the uterine endometrium. Biochem Biophys Res Commun. 1997;234:242–246. doi: 10.1006/bbrc.1997.6624. [DOI] [PubMed] [Google Scholar]
- Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]
- Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973;31:201–256. doi: 10.1016/s0083-6729(08)60999-1. [DOI] [PubMed] [Google Scholar]
- Reis FM, Ribeiro MF, Maia AL, Spritzer PM. Regulation of human endometrial transforming growth factor beta1 and beta3 isoforms through menstrual cycle and medroxyprogesterone acetate treatment. Histol Histopathol. 2002;17:739–45. doi: 10.14670/HH-17.739. [DOI] [PubMed] [Google Scholar]
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Dimitriadis E, Jones RL, Nie G. Complex regulation of decidualization: a role for cytokines and proteases—a review. Placenta. 2003;24(Suppl A):S76–S85. doi: 10.1053/plac.2002.0928. [DOI] [PubMed] [Google Scholar]
- Schneyer AL, Rzucidlo DA, Sluss PM, Crowley WF., Jr Characterization of unique binding kinetics of follistatin and activin or inhibin in serum. Endocrinology. 1994;135:667–674. doi: 10.1210/endo.135.2.8033815. [DOI] [PubMed] [Google Scholar]
- Schneyer A, Schoen A, Quigg A, Sidis Y. Differential binding and neutralization of activins A and B by follistatin and follistatin like-3 (FSTL-3/FSRP/FLRG) Endocrinology. 2003;144:1671–1674. doi: 10.1210/en.2002-0203. [DOI] [PubMed] [Google Scholar]
- Shimonaka M, Inouye S, Shimasaki S, Ling N. Follistatin binds to both activin and inhibin through the common subunit. Endocrinology. 1991;128:3313–3315. doi: 10.1210/endo-128-6-3313. [DOI] [PubMed] [Google Scholar]
- Tang B, Guller S, Gurpide E. Mechanism of human endometrial stromal cells decidualization. Ann N Y Acad Sci. 1994;734:19–25. doi: 10.1111/j.1749-6632.1994.tb21731.x. [DOI] [PubMed] [Google Scholar]
- Tierney EP, Giudice LC. Role of activin A as a mediator of in vitro endometrial stromal cell decidualization via the cyclic adenosine monophosphate pathway. Fertil Steril. 2004;81(Suppl 1):899–903. doi: 10.1016/j.fertnstert.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Tierney EP, Tulac S, Huang ST, Giudice LC. Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics. 2003;16:47–66. doi: 10.1152/physiolgenomics.00066.2003. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Shimizu A, Kato M, Nishitoh H, Ichijo H, Hanyu A, Morita I, Kimura M, Makishima F, Miyazono K. Growth/differentiation factor-5 induces angiogenesis in vivo. Exp Cell Res. 1997;235:218–226. doi: 10.1006/excr.1997.3664. [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad1, during mouse decidualization. Biol Reprod. 2000;63:1781–1786. doi: 10.1095/biolreprod63.6.1781. [DOI] [PubMed] [Google Scholar]
- Zhao R, Lawler AM, Lee SJ. Characterization of GDF-10 expression patterns and null mice. Dev Biol. 1999;212:68–79. doi: 10.1006/dbio.1999.9326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.