Abstract
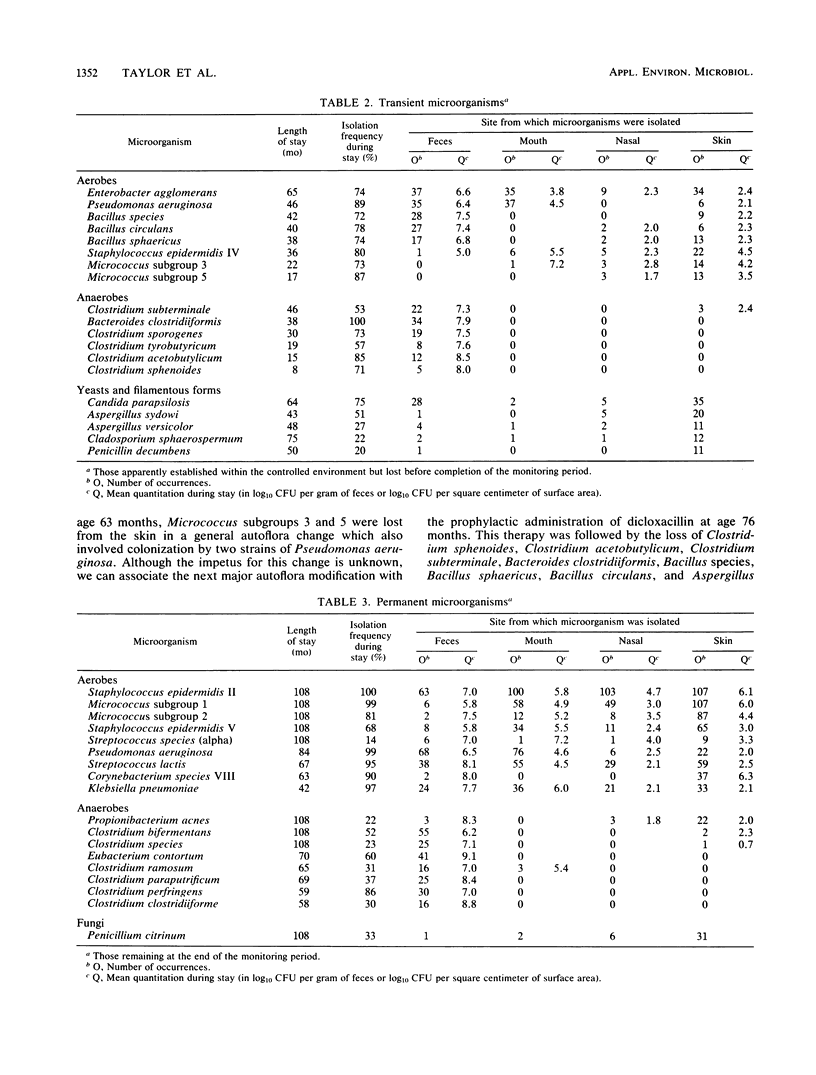
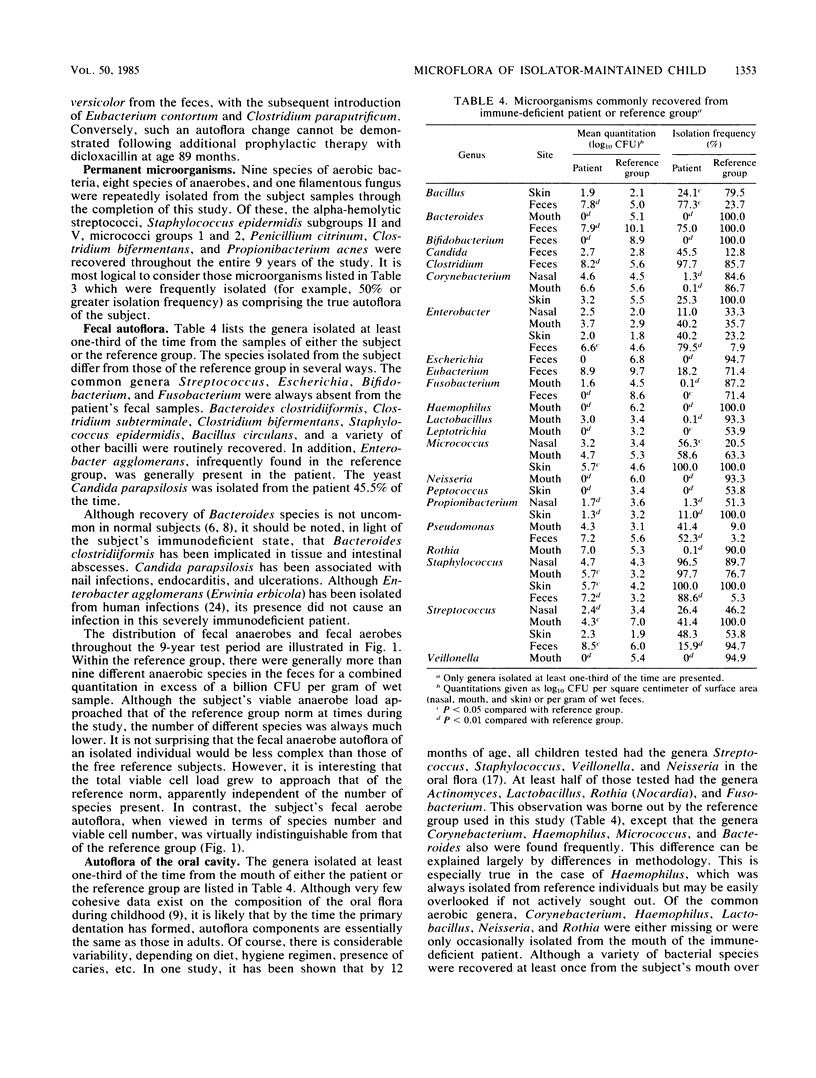
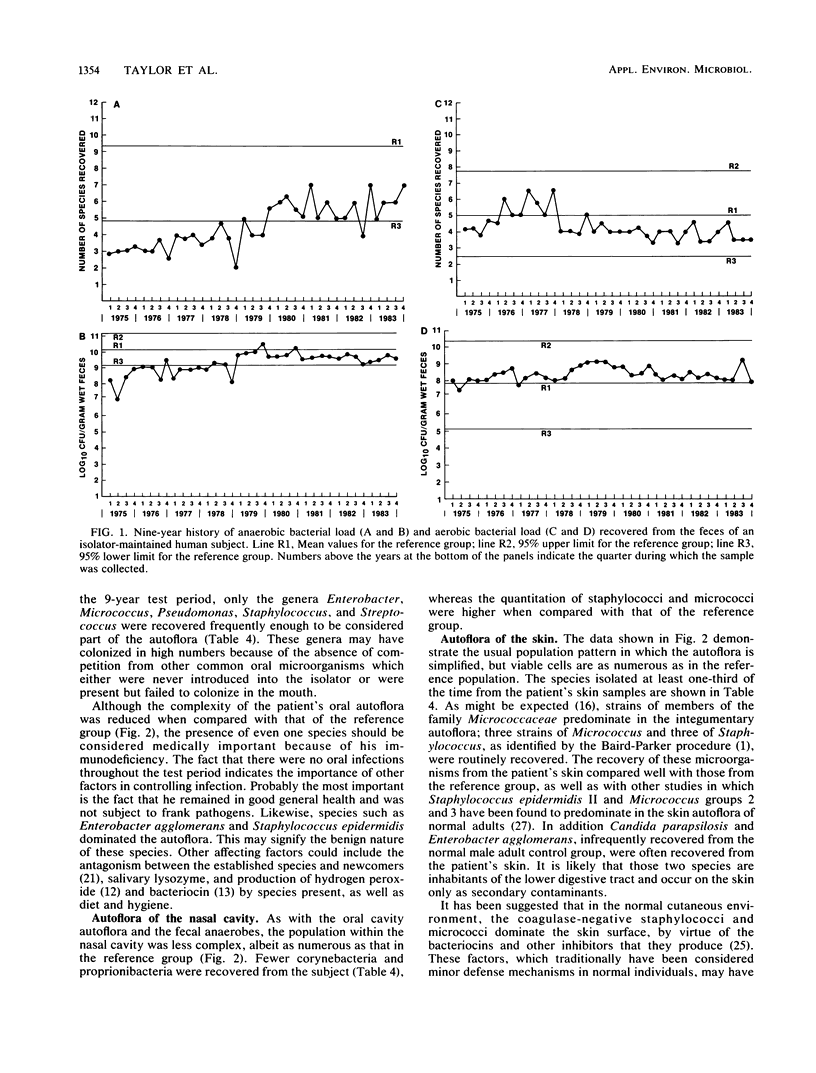
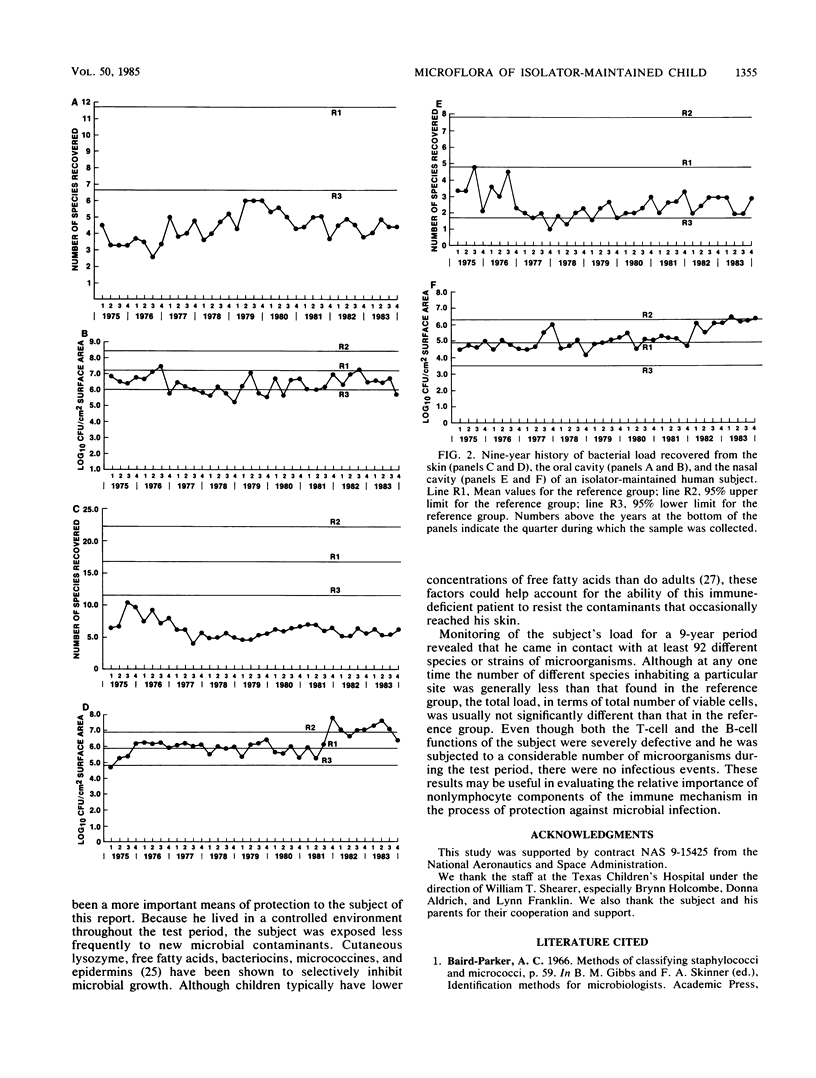
A male child, maintained in a controlled environment, was monitored each month for bacteria, yeasts, and filamentous fungi recovered from the mouth, nasal passages, feces, and nine body surface sites. Three natural microbial categories became apparent. Incident microorganisms were recovered from within the isolator but did not establish permanent residence. Of the 53 incident types isolated, 20 were filamentous fungi and 4 were yeasts. Some genera, such as Fusobacterium, Lactobacillus, Neisseria, and Rothia, which were commonly found in the reference group, did not become permanent inhabitants. Transient microorganisms were repeatedly recovered but could not be demonstrated within the isolated environment at the end of the study. The loss of only a few of the 19 transient species could be associated with antimicrobial therapy. Permanent microorganisms consisted of Pencillium citrinum and 17 bacterial types, of which alpha-hemolytic streptococci, Staphylococcus edpidermidis subgroups II and V, Micrococcus groups 1 and 2, Clostridium bifermentans, and Propionibacterium acnes were recovered throughout the entire 9 years of the study. The number of CFUs recovered from each sample type was generally not unlike that from the reference group of healthy male adults. Also, the number of different aerobic species recovered from the feces was within the normal range of that of the reference group. In contrast, the number of different species recovered from all other samples was less than that commonly found in the reference group.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A special report: four-year study of a boy with combined immune deficiency maintained in strict reverse isolation from birth. Pediatr Res. 1977 Jan;11(1 Pt 2):63–89. doi: 10.1203/00006450-197701000-00001. [DOI] [PubMed] [Google Scholar]
- Carmichael C., Taylor G. R. Evaluation of crew skin flora under conditions of a full quarantine lunar-exploration mission. Br J Dermatol. 1977 Aug;97(2):187–196. doi: 10.1111/j.1365-2133.1977.tb15064.x. [DOI] [PubMed] [Google Scholar]
- De Fazio S. R., Criswell B. S., Kimzey S. L., South M. A., Montgomery J. R. A paraprotein in severe combined immunodefeciency disease detected by immunoelectrophoretic analysis of plasma. Clin Exp Immunol. 1975 Mar;19(3):563–570. [PMC free article] [PubMed] [Google Scholar]
- Decelle J. G., Taylor G. R. Autoflora in the upper respiratory tract of Apollo astronauts. Appl Environ Microbiol. 1976 Nov;32(5):659–665. doi: 10.1128/aem.32.5.659-665.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar B. S., Shiner M., McLeod G. M. Studies on the intestinal flora. I. The bacterial flora of the gastrointestinal tract in healthy and achlorhydric persons. Gastroenterology. 1969 Jan;56(1):71–79. [PubMed] [Google Scholar]
- Finegold S. M. The significance of the intestinal microflora. Del Med J. 1970 Nov;42(11):341–passim. [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. The normal microbial flora of the mouth. Soc Appl Bacteriol Symp Ser. 1974;3(0):47–83. [PubMed] [Google Scholar]
- Henney M. R., Raylor G. R., Molina T. C. Mycological profile of crew during 56-day simulated orbital flight. Mycopathologia. 1978 Aug 10;63(3):131–144. doi: 10.1007/BF00490928. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol. 1973 Mar;18(3):423–434. doi: 10.1016/0003-9969(73)90167-2. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Gibbons R. J. Bacteriocins from human and rodent streptococci. Arch Oral Biol. 1969 Mar;14(3):251–258. doi: 10.1016/0003-9969(69)90227-1. [DOI] [PubMed] [Google Scholar]
- MCCARTHY C., SNYDER M. L., PARKER R. B. THE INDIGENOUS ORAL FLORA OF MAN. I. THE NEWBORN TO THE 1-YEAR-OLD INFANT. Arch Oral Biol. 1965 Jan-Feb;10:61–70. doi: 10.1016/0003-9969(65)90058-0. [DOI] [PubMed] [Google Scholar]
- Mackler B. F., O'Neill P. A. T-lymphocyte induction of non-T cell-mediated nonspecific cytotoxicity. II. A clinical study of a severe combined immunodeficiency patient maintained in a gnotobiotic environment. Clin Immunol Immunopathol. 1979 Mar;12(3):358–366. doi: 10.1016/0090-1229(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Malinak L. R., Wilson R., South M. A., Montgomery J. R., Mumford D. M., Flowers C. E., Jr Germ-free delivery. The initiation of management of infants with a high probability of congenital immune deficiency states. Am J Obstet Gynecol. 1973 May 15;116(2):201–204. doi: 10.1016/0002-9378(73)91051-x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay N., Richie E., Mackler B. F., Montgomery J. R., Wilson R., Fernbach D. J., South M. A. A longitudinal study of T and B lymphocytes from a three-year-old patient with severe combined immunodeficiency (SCID) in 'gnotobiotic protection'. Exp Hematol. 1978 Feb;6(2):129–134. [PubMed] [Google Scholar]
- Mukhopadhyay N., Richie E., Montgomery J., Wilson R., Fernbach D. J., South M. A. Peripheral blood T and B cell characteristics in a patient with severe combined immune deficiency (SCID) maintained in a gnotobiotic environment. Exp Hematol. 1976 Jan;4(1):1–9. [PubMed] [Google Scholar]
- Parker R. B. Paired culture interaction of the oral microbiota. J Dent Res. 1970 Jul-Aug;49(4):804–809. doi: 10.1177/00220345700490041701. [DOI] [PubMed] [Google Scholar]
- Paschall V. L., Brown L. A., Lawrence E. C., Karol R. A., Lotzova E., Brown B. S., Shearer W. T. Immunoregulation in an isolated 12-year-old boy with congenital severe combined immunodeficiency. Pediatr Res. 1984 Aug;18(8):723–728. doi: 10.1203/00006450-198408000-00009. [DOI] [PubMed] [Google Scholar]
- Poplack D. G., Penland W. Z., Levine A. S. Bacteriological isolation garment. Lancet. 1974 Jun 22;1(7869):1261–1262. doi: 10.1016/s0140-6736(74)90012-9. [DOI] [PubMed] [Google Scholar]
- Schneierson S. S., Bottone E. J. Erwinia infections in man. CRC Crit Rev Clin Lab Sci. 1973 Dec;4(4):341–355. doi: 10.3109/10408367309151558. [DOI] [PubMed] [Google Scholar]
- Selwyn S., Ellis H. Skin bacteria and skin disinfection reconsidered. Br Med J. 1972 Jan 15;1(5793):136–140. doi: 10.1136/bmj.1.5793.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer W. T., Ritz J., Finegold M. J., Guerra I. C., Rosenblatt H. M., Lewis D. E., Pollack M. S., Taber L. H., Sumaya C. V., Grumet F. C. Epstein-Barr virus-associated B-cell proliferations of diverse clonal origins after bone marrow transplantation in a 12-year-old patient with severe combined immunodeficiency. N Engl J Med. 1985 May 2;312(18):1151–1159. doi: 10.1056/NEJM198505023121804. [DOI] [PubMed] [Google Scholar]
- Taylor G. R., Henney M. R., Ellis W. L. Changes in the fungal autoflora of Apollo astronauts. Appl Microbiol. 1973 Nov;26(5):804–813. doi: 10.1128/am.26.5.804-813.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R., Kropp K. D., Molina T. C. Microflora analysis of a child with severe combined immune deficiency. Infect Immun. 1978 Feb;19(2):385–390. doi: 10.1128/iai.19.2.385-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R. Recovery of medically important microorganisms from Apollo astronauts. Aerosp Med. 1974 Aug;45(8):824–828. [PubMed] [Google Scholar]
- Taylor G. R., Zaloguev S. N. Medically important micro-organisms recovered from Apollo-Soyuz Test Project (ASTP) crew members. Life Sci Space Res. 1977;15:207–212. [PubMed] [Google Scholar]


